INTRODUCTION
Peripheral nerve injuries are commonly encountered clinical problem and often result in chronic pain and severe functional deficit. The affected limb displays characteristics of painful neuropathy, such as hyperalgesia, pain related gait, and swelling. Ascending spinal pathways subsequently distribute nociceptive information to multiple cortical, limbic structures and hypothalamus involved in the pain (Hunt and Mantyh, 2001). Transmission of nociceptive information may be altered by many neuronal circuits within the central nervous system. The periaquaductal gray (PAG) and paraventricular nucleus (PVN) are an important sites in ascending pain transmission.
c-Fos, an immediate early gene, is used as a marker for stimuli-induced metabolic changes of neurons, and its expression is also induced under various conditions (de Medeiros et al., 2003). c-Fos expression is recognized as a marker of increased neuronal activity (Lee et al., 2003). Sciatic nerve ligation increased c-Fos expression in the frontal cortex, thalamus, and PAG in rats (Narita et al., 2003).
The brain-derived neurotrophic factor (BDNF) is an important neuromodulator of synaptic plasticity in the peripheral nerve injury, and it is a member of the neurotrophic family that promotes the survival of specific neurons during development (Woolf and Costigan, 1999). It has higher affinity for the receptor tyrosine kinase B (TrkB) (Maness et al., 1994).
Prostaglandins (PGs) are key inflammatory mediators that are converted from arachidonic acid by cyclooxygenase (COX). There are two isoforms of COX: cyclooxygenase-1 (COX-1) is constitutively expressed in nearly all tissue and provides PGs to maintain physiological functions, such as cytoprotection of the stomach and regulation renal blood flow (Vane et al., 1998). COX-2 plays a major role in the biosynthesis of PGs from arachidonic acid, and COX-2 is upregulated in the injured nerve by partial sciatic nerve ligation (Ma and Eisenach, 2002, 2003).
Nitric oxide (NO) is a messenger and effecter molecule in a variety of tissues (Lowenstein and Greenberg, 1996). NO was identified as a neurotransmitter, and it acts as a potent vasodilator in physiological condition (Baek et al., 2016). The production of NO is regulation by intracellular nitric oxide synthases (NOS), and three types of NOS have been identified: endothelial nitric oxide synthase (eNOS), neural nitric oxide synthase (nNOS), and inducible nitric oxide synthase (iNOS). Of these, iNOS is implicated in the pathophysiology of inflammatory and neuropathic pain (De Alba et al., 2006). iNOS expressed by glia is involved in the mechanisms of hyperalgesia.
Diosgenin is a steroidal saponin, which is found in several plant species, and particularly in fenugreek or wild yam (Dioscorea spp.) roots. Plant saponin has anti-inflammatory, anti-oxidative, and anticancer effects (Raju et al., 2004). Au et al. (2004) reported that diosgenin is structurally similar to progesterone and estrogen, and diosgenin caused acute endothelium-independent coronary artery relaxation. Saponin-rich plant acts as an anti-inflammatory agent through inhibiting COX-2 and iNOS expressions, thereby inhibiting production of PGs (Li et al., 2006). Moharram and El-Shenawy (2007) and Wang et al. (2008) also reported that plant saponin exhibited anti-nociceptive action.
The sciatic function index (SFI) compares parameters from the normal and experimental footprints by a mathematical formula, and provides information concerning the recovery of sensory-motor connections and cortical integration related to gait function mediated by the sciatic nerve (Byun et al., 2005).
In the present study, we investigated the effect of diosgenin on sciatic crushed nerve injury in rats. For this, walking track analysis for the functional recovery which can be quantified with the SFI was conducted. Immunohistochemistry for the c-Fos expression in the ventral lateral PAG (vlPAG) and PVN and western blot analysis for the expressions of BDNF, TrkB, COX-2, and iNOS in the sciatic nerve were conducted.
MATERIALS AND METHODS
Animals and treatments
Male Sprague-Dawley rat weighing 350±10 g (12 weeks of age) were used. The experimental procedures were performed in accordance with the animal care guideline of National Institutes of Health and the Korea Academy of Medical Sciences. The rats were randomly divided into five groups (n=10 in each group): the shame operation group, the sciatic crushed nerve injury group, the sciatic crushed nerve injury and 25 mg/kg diosgenin-treated group, the sciatic crushed nerve injury and 50 mg/kg diosgenin-treated group, and the sciatic crushed nerve injury and 100 mg/kg diosgenin-treated group. The rats in the diosgenin-treated groups received diosgenin (Wako Pure Chemical Industries Ltd., Osaka, Japan) orally once a day for 7 consecutive days, starting one day after surgery.
Surgical procedure
To induce crush injury on the sciatic nerve, the previously described surgical procedure was performed (Byun et al., 2005). Right sciatic nerve was exposed by incision on the gluteal muscle under anesthesia using Zoletil 50 (50 mg/kg; Virbac Laboratories, Carros, France). The sciatic nerve was carefully exposed and crushed for 30 sec using a surgical clip (pressure: 125 g; Fine Science Tool Inc., San Francisco, CA, USA). The crushed location was between the sciatic notch and the point of trifurcation.
Walking tract analysis
Functional recovery rate after nerve injury was analyzed using a walking tract assessment, as the previously described method (Byun et al., 2005). From the footprints the following parameter were calculated: distance from the heel to the top of the 3rd toe (print length, PL), distance between the 1st to the 5th toe (toe spread, TS), and distance from the 2nd to the 4th toe (intermediary toe spread, IT). These parameters were taken both from the intact left (non-operated) foot (NPL, NTS, and NIT) and from the injured right (experimental) foot (EPL, ETS, and EIT). SFI values were obtained using following equation: SFI=(−38.3±PLF)+ (109.5±TSF)+(13.3±ITF)–8.8. Print length factor (TSF)=(ETS–NPL)/NPL. Toe spread factor (TSF)=(EST–NTS)/NTS. Intermediary toe spread factor (ITF)=(EIT–NIT)/NIT. Interpolating identical values of PL, TS, and IT from the right and left hind feet are close to zero in normal rats. The SFI value of −100 indicates complete impairment.
Tissue preparation
The animals were sacrificed on 7 days after treatment. The animals were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Virbac Laboratories), transcardially perfused with 50 mM phosphate-buffered saline, and fixed with a freshly prepared solution consisting of 4% parafomaldehyde in 100 Mm phosphate buffer (pH, 7.4). The brains were dissected and postfixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Coronal section of 40-μm thickness was made with a freezing microtome (Leica, Nussloch, Germany).
c-Fos immunohistochemistry
c-Fos immunohistochemistry was performed as the previously described method (Han et al., 2017). Free-floating tissue sections were incubated overnight with rabbit anti-c-Fos antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and the section were then incubated for 1 hr with biotinylated anti-rabbit secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were subsequently incubated with avidin-biotin-peroxidase complex (1:100; Vector Laboratories) for 1 hr at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% 3,3-diaminobenzidine and 0.01% H2O2 in 50 mM Tris-buffer (pH, 7.6) for approximately 3 min. The slides were air-dried over night at room temperature, and coverslips were mounted using Permount (Fisher Scientific, Pittsburgh, PA, USA).
Western blot analysis
Western blot analysis for BDNF, TrkB, COX-2, iNOS were performed according to the previous method (Baek et al., 2016; Park et al., 2017). Sciatic nerve tissue samples were homogenized in the lysis buffer and 200 μg the samples were centrifuged at 14,000 rpm at 20 min and supernatant collected for Western blot. Protein concentration in the sample was assayed using Bradford reagent (Bio-Rad Laboratories Inc., Hercules, CA, USA). Protein of 35 μg was separated sodium dodecyl sulfate-polyacrylamide gels and transferred to nitrocellulose membrane (Whatman GmbH, Dassel, Germany). Rabbit BDNF antibody (1:1,000; Santa Cruz Biotechnology), rabbit TrkB antibody (1:1,000; Santa Cruz Biotechnology), goat COX-2 antibody (1:1,000; Santa Cruz Biotechnology), and rabbit iNOS antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody (1:2,000; Vector Laboratories) for BDNF, TrkB, iNOS, and anti-goat antibody (1:2,000; Santa Cruz Biotechnology) for COX-2 were used as the secondary antibodies. Band detection was performed using an enhanced chemiluminescence detection system (Santa Cruz Biotechnology).
RESULTS
SFI following sciatic crushed nerve injury
The mean SFI for each group was calculated on the 3rd, 5th, and 7th day after sciatic crushed nerve injury. The present results indicated that diosgenin treatment promoted functional locomotor recovery following sciatic crushed nerve injury (Fig. 1).
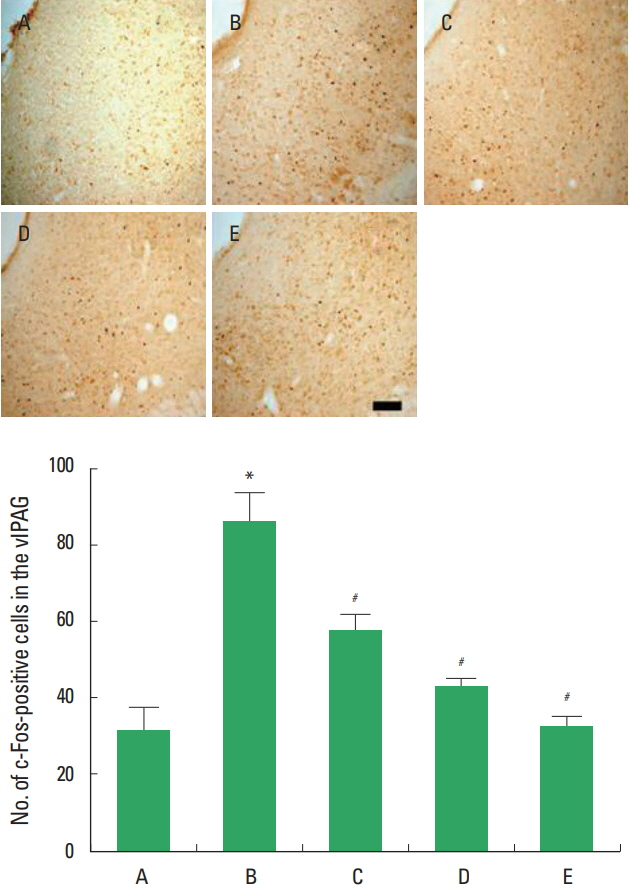
c-Fos expression in the vlPAG following sciatic crushed nerve injury
The present results showed that sciatic crushed nerve injury enhanced c-Fos expression in the vlPAG and diosgenin treatment decreased c-Fos expression in the vlPAG (Fig. 2).
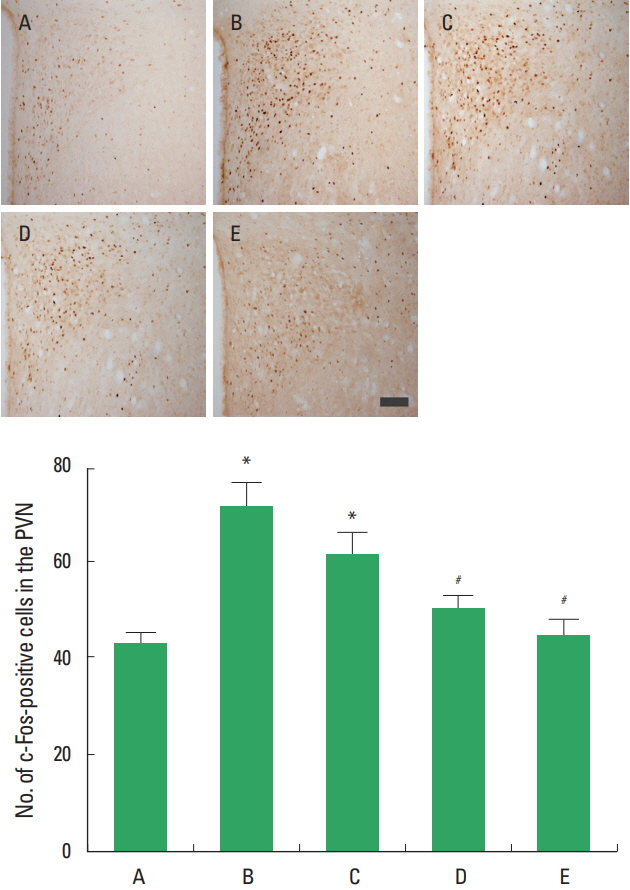
c-Fos expression in the PVN following sciatic crushed nerve injury
The present results showed that sciatic crushed nerve injury decreased c-Fos expression in the PVN and diosgenin treatment enhanced c-Fos expression in the PVN (Fig. 3).
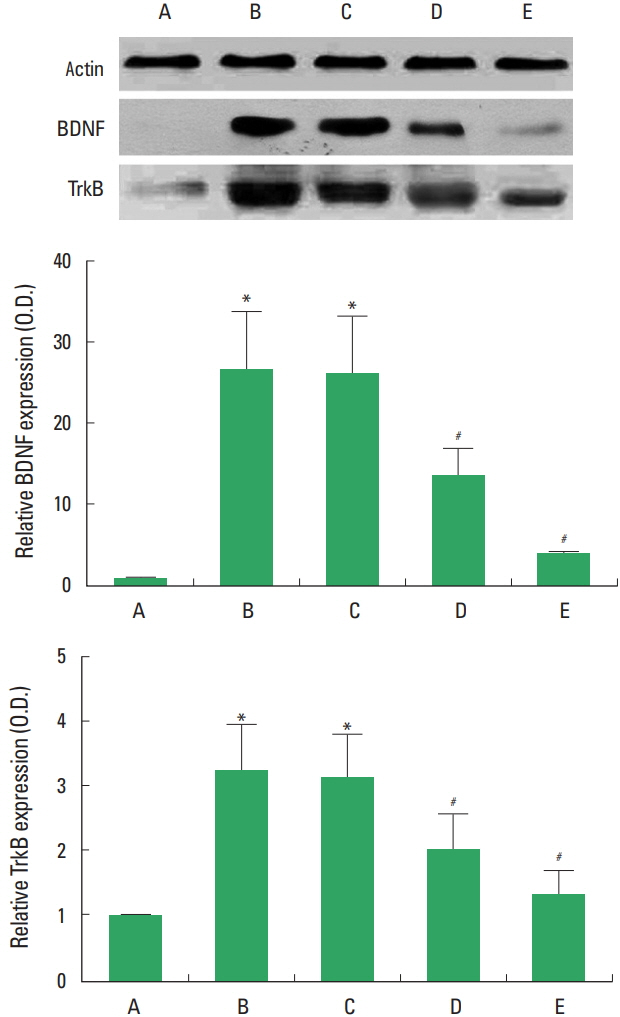
BDNF and TrkB expressions in the sciatic nerve following sciatic crushed nerve injury
The present results showed that sciatic crushed nerve injury increased the levels of BDNF and TrkB, while treatment with diosgenin decreased the levels of BDNF and TrkB (Fig. 4).
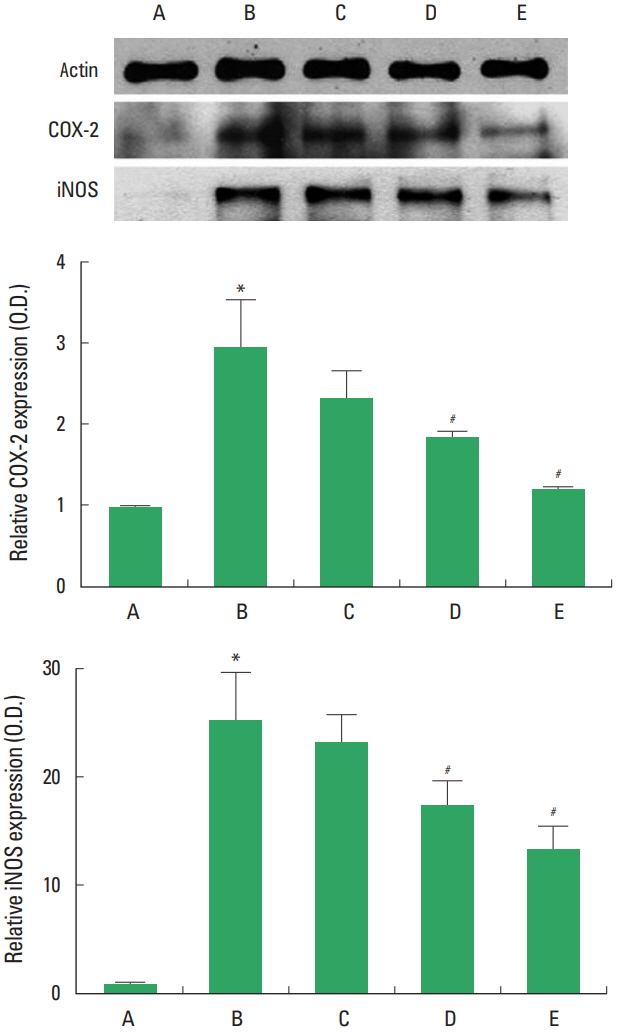
COX-2 and iNOS expressions in the sciatic nerve following sciatic crushed nerve injury
The present results showed that decreased the levels of COX-2 and iNOS, while treatment with diosgenin decreased the levels of COX-2 and iNOS (Fig. 5).
DISCUSSION
The SFI is widely used to evaluate functional gait assessment after peripheral nerve lesion (Byun et al., 2005). In the present study, sciatic nerve crushed injury showed decrease of the SFI value. However treatment with diosgenin increased SFI value, indicting that diosgenin increased functional recovery following sciatic crushed nerve injury.
c-Fos is a sensitive maker of the neuronal activation following sciatic nerve ligation (Nishimori et al., 2002). c-Fos expression is rapidly and transiently induced in the brain areas in response to nerve stimulation (Han et al., 2017). In the present study, c-Fos expression in the vlPAG and PVN was increased following sciatic crushed nerve injury, while diosgenin treatment suppressed the nerve injury-induce c-Fos expression in the vlPAG and PVN. The present results indicate that neurons in the vlPAG and PVN were activated by sciatic crushed nerve injury. In contrast, diosgenin suppressed noxious stimulation on sciatic nerve, resulting in decrease of neuronal activation in the vlPAG and PVN.
Neuronal BDNF expression was rapidly and transiently increased in response to peripheral nerve injury (Cho et al., 1997). Woolf and Costigan (1999) reported that BDNF may play a critical role in synaptic plasticity in nociceptive signaling. Overexpression of BDNF by sciatic nerve injury restrained recovery of locomotor function and treadmill exercise promoted functional recovery rate via suppression on BDNF mRNA expression (Byun et al., 2005). In the present study, BDNF expression in the sciatic nerve was increased by sciatic crushed nerve injury, suggesting that injury to the sciatic nerve induced BDNF expression for its regeneration. In contrast, diosgenin treatment suppressed the nerve injury-induced BDNF expression, suggesting that diosgenin facilitated regeneration of injury to the sciatic nerve, thus overexpression of BDNF was suppressed.
COX-2 causes PGE2 release in response to surgical trauma, and COX-2 is implicated in inflammation and pain (Baba et al., 2001). Peripheral inflammation up-regulates COX-2 expression in spinal cord with subsequent increase in PGE2 level, and results in development of allodynia and hyperalgesia (Guay et al., 2004). Alba et al. (2006) demonstrated that iNOS expression was overexpressed under pain conditions, and iNOS inhibitors exerted anti-inflammatory effect. Therefore, expressions of COX-2 and iNOS are closely associated with the pathogenesis of inflammatory and neuropathic pain (Baek et al., 2016). In the present study, COX-2 and iNOS expressions were increased by sciatic crushed nerve injury, indicating that injury to the sciatic nerve induced inflammation. In contrast, treatment with diosgenin inhibited COX-2 and iNOS expressions in sciatic nerve injury, representing that diosgenin exerted anti-inflammatory effect on sciatic crushed nerve injury.
Diosgenin has a large variety of biological functions, such as anti-oxidative, anticancer and anti-inflammatory effects (Moalic et al., 2001; Shishodia and Aggarwal, 2006). In the present study, diosgenin facilitated functional recovery from sciatic crushed nerve injury, thus showed suppression on BDNF and its receptor TrkB, resulted in decrease of neuronal activity in the vlPAG and PVN. Based on the present result, diosgenin can be used as the new therapeutic agent for pain control and functional recovery following peripheral nerve injury.