Effect of bodybuilding athletes’ weight loss method on performance factors and immune function
Article information
Abstract
This study was conducted on elite bodybuilders for 6 weeks using two weight loss methods (traditional method=high-weight loss vs. new method=low-weight loss). The subjects of this study were 26 male active elite bodybuilders who voluntarily wanted to participate in this experiment, and were divided into experimental group and traditional group. The traditional and experimental groups underwent dietary restrictions and training for 6 weeks. The conclusion obtained from the results of this study is that in the case of anthropometric variables, there is a difference in weight class between the two groups. The experimental group’s upper arm circumference was maintained compared to the pre-measured value after post-measurement, while the traditional group decreased, and a significant interaction effect was observed. In particular, a significant interaction effect was observed. In that the absolute value of maximum oxygen intake was significantly increased only in the post-experimental group compared to the pretest, and an interaction effect was observed, the use of low-weight loss method is more effective than the traditional method of high-weight loss method. An increase in some cytokines was observed despite traditional weight loss, but it did not cause an excessive decrease in immunity or a sharp decrease in performance factors. As a result of this experiment, it is judged that the use of a low-weight loss method is more preferable than the traditional high-weight loss method in relation to performance variables.
INTRODUCTION
The goal of bodybuilding athletes to improve their performance is to develop maximum muscle hypertrophy (size) and definition based on a balanced body type (Lambert et al., 2004; Ravaldi et al., 2003). To achieve this, muscle mass must be maximally increased during the off-season, and body fat must be reduced to the lowest limit in the precompetition period to increase muscle separation and definition while maintaining maximal muscle mass. In these two phases, in the off-season hypertrophy phase, which is the initial phase, a greater amount of energy is consumed to hypertrophy the muscles than the athlete’s usual energy intake to maintain his/her weight class, and in the second phase, before the competition, In the muscle separation and definition phase, rapid weight loss is performed to return the weight gained from the muscle hypertrophy phase to the original weight class.
Nonprofessional performers in sports that emphasize thinness and musculature, such as ballet and bodybuilding, have high levels of body anxiety and inappropriate eating habits and behaviors (Ravaldi et al., 2003). Elite-level bodybuilders are known to lose an average of about 12% of their body weight over a period of at least 3 to 10 weeks prior to competition (Manore et al., 1993). In Korea, weight loss of about 15% is traditionally carried out on average over a similar period of time (about 3–8 weeks). In the case of some athletes with excellent performance, unlike the traditional high-weight loss method (approximately 15% weight loss), they are using a low-weight loss method of 5%–6% over the same period as the traditional weight loss method.
Meanwhile, athletes in combat sports such as judo, taekwondo, and wrestling are known to attempt to lose weight in a relatively short period of time compared to bodybuilding athletes (Artioli et al., 2010). Approximately 70% of wrestlers attempt short-term weight loss within 7 days, and the remaining 30% report gradual weight loss over 7 days or more prior to competition (Kordi et al., 2011), and boxing and judo athletes. It is also reported that wrestlers lose a similar amount of body weight (about 10%) over a similar period of time (Oppliger et al., 2003). However, nutritional deficiencies during weight loss are reported to lower resistance to infection (Chandra, 2002), and even short-term weight loss of less than 2–3 weeks leads to a decrease in immunity (Suzuki et al., 2003) and muscle damage that leads to a decrease in athletic performance. It has been reported that mass loss, temperature regulation difficulties, and reduced oxygen capacity can have fatal adverse effects on the health of athletes (Ransone and Hughes, 2004).
Unlike bodybuilding, speculative sports attempt to lose weight primarily through dehydration and reduction of total calorie intake through rapid weight loss over a relatively short period of time (less than 2 weeks). However, bodybuilders lose weight over a relatively long period of time (more than 3 weeks), and during the weight loss period, total calorie intake is drastically reduced, but protein intake is maintained to maintain muscle mass. It has been reported that intake of micronutrients that strengthen the body’s immune function through supplements exceeds the standard (Florentino, 2009). To date, there has been no comparative/analytical research on the physiological effects and performance improvement effects of traditional weight loss methods (approximately 15%) and new weight loss methods (approximately 5%–6%), and scientific verification is needed.
MATERIALS AND METHODS
The subjects of this study were 26 male active elite bodybuilders belonging to the Bodybuilding Association who had won prizes in national competitions and were willing to voluntarily participate in this experiment. Among the athletes wishing to participate in the experiment, 13 were selected as the high-weight loss method (traditional group), and 13 athletes practicing low-weight loss method (experimental group) were selected as the experimental group. After going through a period of muscle hypertrophy (bulking up), they implemented a weight loss method through dietary restrictions for 6 weeks. This study was approved by Eulji University (approval number, 2022-3-215).
Blood analysis
After arriving at the laboratory, the subjects rested for 30 min and then their body weight and resting heart rate were measured. 20 mL of blood was collected for measurement and analysis of blood variables before and after the experiment. Blood was collected in both groups on a 12-hr fast. The collected blood was centrifuged at 3,000 rpm for about 10 min using a centrifuge, and the serum was stored at −80°C. The stored serum was analyzed for immunoglobulin (Ig) A, IgG, IgM, and cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6.
Maximum oxygen intake measurement
Using an automatic gas analyzer, maximum oxygen intake (VO2max), respiratory exchange rate, heart rate, and anaerobic threshold were measured using a treadmill maximum incremental exercise load test method. The initial starting exercise load was a speed of 80 m/min and a slope of 6%, and then the slope was fixed at 6% and the speed was increased by 20 m/min every 2 min. A test was conducted until the subject reached all-out.
Measurement of maximal strength
Maximal strength (one-repetition maximum, 1-RM) was measured using an indirect measurement method using the Lombardi coefficient. This is a method that can estimate 1-RM using only one-repetition using a selected random weight, and the error with the 1-RM measurement value by the direct method is very small, so it is a highly reliable method and is widely used in sports fields.
Body measurements
The subjects’ muscle circumference was measured in the pectoralis major, brachialis, waist, and thigh muscles, and the unit of measurement was 0.5 cm. Body weight was measured to the nearest 0.1 kg using an electronic scale. Body composition was measured using a body composition analyzer (Inbody 720, Biospace, Seoul, Korea) for 6 weeks.
Types and specific methods of exercise
The exercise experiment conducted in this study consisted of resistance training (free weights) and aerobic exercise using a treadmill. The same training was performed under conditions appropriate for 1-RM and VO2max values considering each individual’s weight class and physical strength level. Six weeks of weight training and six weeks of aerobic exercise were performed. In this study, the only difference was the weight loss method through dietary intake restriction (experimental group=low-weight loss vs. traditional group=high-weight loss), and the same training program was applied to both groups.
Data processing
Data processing for this study used the SPSS ver. 12.0 (SPSS Inc., Chicago, IL, USA) statistical program to calculate the mean and standard deviation for all variables. Differences in blood variables, VO2max, and muscle strength between the two groups (low-weight loss vs. high-weight loss) by period (pre, post, or 6 weeks) were analyzed using repeated measure analysis of variance. Bonferroni was used as a post hoc test method, and all statistical significance levels (α) in this study were set at 0.05.
RESULTS
Changes in anthropometric variables according to weight loss method
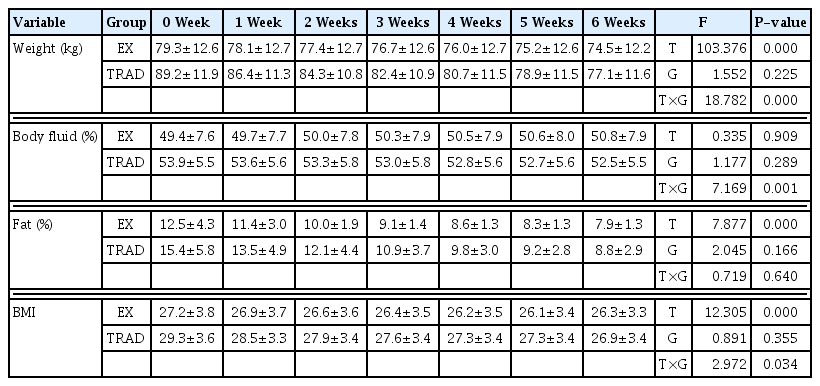
Traditional weight loss method showed a weight loss rate of 16%±4%. The experimental group that tried the new weight loss method, low-weight loss method, showed an average loss rate of about 6.5%±2%. An interaction effect was found in weight, body mass index (BMI), and body water, while a significant difference was found in body fat percentage (%) only by period (Table 1).
Changes in pre- and postperformance factors according to weight loss method
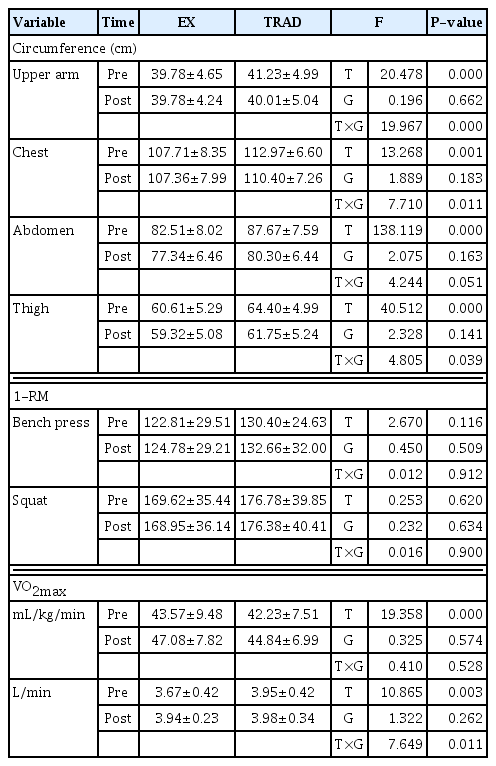
Interaction effects were found only for upper arm circumference, chest circumference, thigh circumference, and VO2max absolute value (L/min) excluding abdominal circumference, while significant differences were found only between periods for abdominal circumference and VO2max relative value (mL/kg/min). There was no significant difference in the 1-RM of bench press and squat in both the main effect and interaction effect. In other words, as variables of change in human body circumference and performance, upper arm circumference, chest circumference, thigh circumference, and VO2max absolute value (L/min), excluding bench press, squat, and VO2max peak value (mL/kg/min), vary depending on the degree of weight loss. It was confirmed that an interaction effect occurred due to the difference in the patterns of these variables. In other words, the experimental group’s upper arm circumference, chest circumference, and thigh circumference were maintained or decreased very gently, while the traditional group’s upper arm circumference, chest circumference, and thigh circumference showed a sharp decrease. On the other hand, in the case of abdominal circumference, both groups showed the same pattern of rapid decrease, with significant differences only appearing over time. In the case of bench press and squat 1-RM, both groups remained almost constant. Meanwhile, the relative value of VO2max was observed to increase due to weight loss in both groups after compared to before, while the absolute value of VO2max (L/min) in the experimental group increased sharply after compared to before in the experimental group, while it remained constant in the traditional group. A pattern difference was maintained. It was found that this difference in pattern caused an interaction effect (Table 2).
Changes and analysis results in pre- and postblood variables according to weight loss method
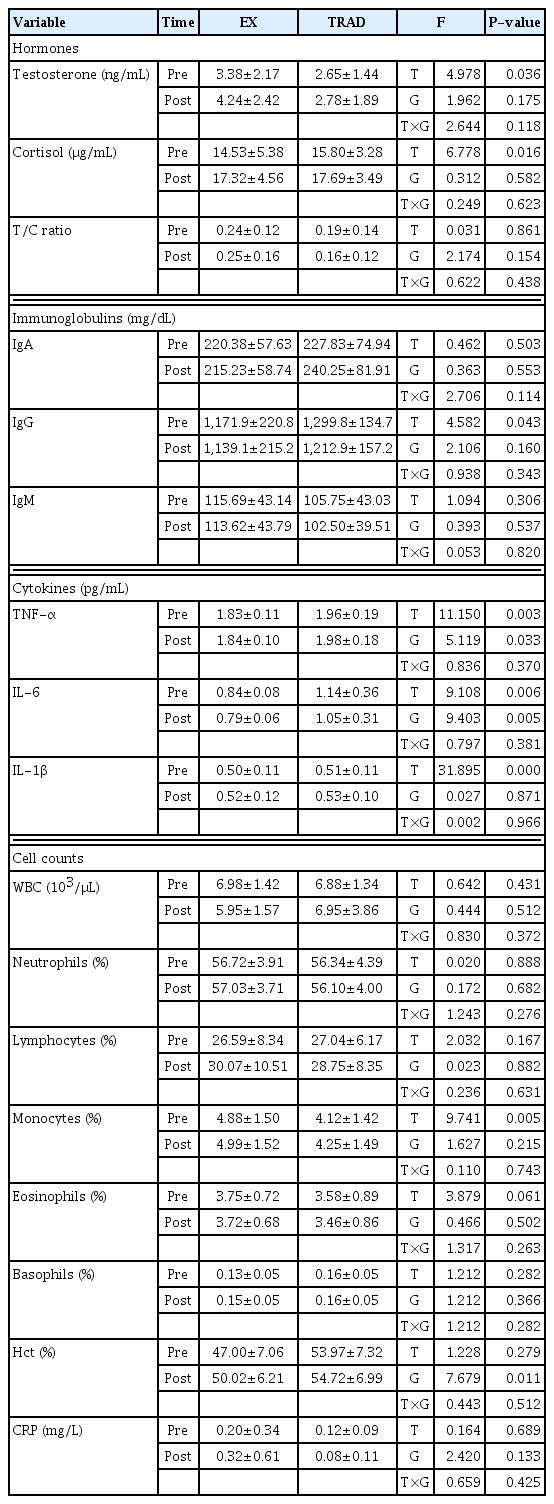
In testosterone (T), cortisol (C), and T/C ratio observed as hormonal variables, only differences between periods were observed between T and C. As a result of post hoc analysis, both T (P=0.036) and C (P=0.016) significantly increased in the post period compared to the preperiod only in the experimental group. There was no statistically significant difference in the T/C ratio, but the experimental group showed a tendency to increase compared to the dictionary, while the traditional group showed a decreasing trend, contrary to the experimental group. Among IgA, IgG, and IgM observed with immunoglobulin, only IgG showed a significant decrease postmortem compared to premortem. As a result of post hoc analysis, a decrease in IgG was found only in the traditional group (P=0.043). In TNF-α, IL-6, and IL-1β observed as cytokine variables, significant differences were observed for TNF-α in both groups (P=0.033) and time (P=0.003), whereas for IL-1β, both groups showed significant differences. It was found that there was a significant increase (P=0.000) in the post period compared to the preperiod. Among these subunits, including white blood cell, only monocytes showed a significant increase (P=0.005) after compared to before. As a result of post hoc analysis, this increase was observed only in the experimental group. Meanwhile, in C-reactive protein (CRP), there were no significant differences in the period, group, and interaction effects (Table 3).
DISCUSSION
In this study, the experimental group’s weight and BMI decreased tended to be very gradual, whereas the traditional group’s weight and BMI decreased at a very steep slope over 6 weeks. In the case of body water, the traditional group showed a gradual decrease over the 6-week weight loss period, while the experimental group showed a gradual increase in the pattern. However, in the case of body fat percentage, significant differences were observed only during various periods, and both groups decreased over 6 weeks regardless of the degree of weight loss. In the case of speculative sports, most lose weight within 2–3 weeks on average to achieve a weight loss of about 10% of their body weight, and 70% of them perform rapid short-term weight loss within 7 days (Artioli et al., 2010; Oppliger et al., 2003). In contrast, bodybuilders have the characteristic of long-term weight loss rather than short-term weight loss. In order to lose about 15% of body weight, more than half (55%) of bodybuilders report restricting their diet 2 to 4 months before competition (Kleiner et al., 1994). In conclusion, it is judged that a new weight loss method that reduces the amount of weight loss is more effective in terms of increasing or preserving muscle mass than using traditional weight loss methods.
The upper arm circumference, chest circumference, and thigh circumference of the experimental group were maintained during the weight loss period, while the abdominal circumference significantly decreased. However, in the case of the traditional group, the upper arm, chest, abdomen, and thigh circumferences all decreased significantly. There was no significant difference in bench press and squat 1-RM in both groups. These differences in changes in major muscle circumference between groups can be interpreted as being influenced by the degree of dietary intake restriction. In addition, it is believed that the difference in weight between the two groups (75 kg less vs. 75 kg or more) caused the difference in postmortem circumference of major muscle areas. Bouchard et al. (1990) stated that resistance training to increase muscle strength is effective in reducing body fat when repeated multiple times at low intensity. This suggests that the low-weight loss method in the 6±2% range did not cause an improvement in muscle strength, but could minimize or maintain changes in muscle circumference or muscle mass. Excessive weight loss among athletes places great burden on athletes, regardless of their sport. If low-weight loss can minimize body fat percentage while maintaining muscle circumference or mass similar to high-weight loss, low-weight loss is considered to be a much more effective and less burdensome method for athletes. Therefore, low-weight loss can be used as an important means of improving the performance of bodybuilders.
While it has been suggested that resistance exercise has a positive effect on the immune system (Tokmakidis et al., 2004), resistance exercise does not have a positive effect on immune function and training may sensitize the body to viral infections (Brenner et al., 1994), suggesting conflicting results. In this study, there was no statistically significant difference in the T/C ratio, but the experimental group showed an increasing trend compared to the dictionary, while the traditional group showed a decreasing trend, contrary to the experimental group. According to Petersen and Pedersen (2005), TNF-α is produced in adipocytes, TNF-α stimulates the production of IL-6 in adipocytes and blood monocytes, and IL-6 regulates the concentration of IL-1β and CRP increases. In other words, these cytokines are closely related to adipocytes, and in the case of ordinary people with a relatively high body fat percentage compared to bodybuilders, a decrease in body fat percentage through regular exercise can cause a decrease in these cytokines. Among IgA, IgG, and IgM observed with immunoglobulin, only IgG showed a significant decrease in post compared to pre, and as a result of post hoc analysis, a decrease in IgG was found only in the control group (P=0.043). In the case of TNF-α, a significant difference was found in both group (P=0.033) and period (P=0.003), while IL-1β showed a significant increase (P=0.000) in post both groups after compared to pre.
Despite traditional weight loss, an increase in some cytokines was observed, but it did not cause an excessive decrease in immunity or a sharp decrease in performance factors. As a result of this experiment, it is judged that the use of a low-weight loss method is more preferable than the traditional high-weight loss method in relation to performance variables.
ACKNOWLEDGMENTS
This paper was supported by Eulji University in 2023.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.