Improved arterial stiffness after combined aerobic and resistance training: correlation with heart rate variability change in prehypertensive offspring of hypertensive parents
Article information
Abstract
Prehypertensive offspring of hypertensive parents are strongly linked to pathological processes of hypertension in later life. It is observed that young adults with high blood pressure (BP) have increased arterial stiffness, which is linked to autonomic anomalies. The purpose of the present study was to assess the effect of combined resistance and aerobic exercise training on BP, brachial-ankle pulse wave velocity (baP-WV), and heart rate variability (HRV) in prehypertensive young men with hypertensive parents. Thirty prehypertensive males aged 19.6±1.2 years were randomly assigned to either a combined exercise training group (CBT, n=15) or no exercise group (CON, n=15). The CBT group performed combined exercise for 8 weeks, 3 times per week. BP, baP-WV, HRV, peak oxygen consumption (VO2peak), and muscle strength were measured before and after the exercise intervention. Systolic BP (−5.1 mmHg, 3.9% decrease, P<0.05), diastolic BP (−3.9 mmHg, 6.1% decrease, P<0.01), mean arterial pressure (−4.2 mmHg, 4.7% decrease, P<0.05), baPWV (−0.4 m/sec, 3.5% decrease, P<0.01), standard deviation of all RR intervals (+58.5% increase, P<0.05) and very low frequency (VLF) (+34.6% increase, P<0.01), VO2peak (+11.7% increase, P<0.01) and one-repetition maximum leg press (+30.3% increase, P<0.01) were significantly improved in the CBT group after 8 weeks of training versus the CON group. Additionally, a reduction in baPWV was associated with an increase in the VLF power of HRV (r=0.72, P<0.01). These findings indicate that the combined training improves BP, arterial stiffness, vagal activity, cardiorespiratory fitness, and lower-body muscle strength in prehypertensive offspring of hypertensive parents.
INTRODUCTION
Prehypertension is a global issue that has emerged as one of the most serious diseases threatening human health, especially among young adults (Senthil and Krishndasa, 2015). Prehypertension affects approximately 33% of Thais aged 15 and older, with a higher prevalence among men than women (Aekplakorn et al., 2008). Prehypertension increases the risk of developing clinical hypertension (HT) and, as a result, cardiovascular disease (CVD) (Chobanian et al., 2003). Individuals with prehypertension are more likely than normotensive people to develop essential HT and cardiovascular events (Egan and Stevens-Fabry, 2015). Furthermore, there is evidence that autonomic abnormalities, such as increased sympathetic modulation or decreased vagal activity, may contribute to the development of HT in normotensive offspring of hypertensive parents (Francica et al., 2013; Lénárd et al., 2005). Furthermore, vascular abnormalities have been considered as possible causes of HT in this population (Boutcher et al., 2009; Evrengul et al., 2012).
Arterial stiffness contributes to elevated blood pressure (BP), which can lead to overt HT. Brachial-ankle pulse wave velocity (baPWV), an index of systemic arterial stiffness, has been linked to high BP from adolescence until old age (Zebekakis et al., 2005). Individuals with high BP are more likely to have autonomic dysfunction. A greater sympathovagal balance is known to increase vasoconstriction (Sheng and Zhu, 2018), which may have an effect on PWV (Ghiadoni et al., 2009). Heart rate variability (HRV) is used to assess autonomic imbalances, diseases, and mortality (Galinier et al., 2000). Reduced HRV is associated with an increased risk of cardiac events and death in prehypertensive individuals (Zhao et al., 2021). In hypertensive patients, HRV is reduced, and reduced HRV is one of the most powerful predictors of death (Dekker et al., 1997). Consequently, reducing arterial stiffness, autonomic imbalance, and BP can be therapeutic in decreasing the incidence of CVD in young prehypertensive men. The prevention of HT in young prehypertensive individuals is a major public health priority.
Regular exercise has been demonstrated to reduce arterial stiffness in normotensive and hypertensive adults (Li et al., 2015) and improve autonomic cardiac imbalance in young and early middle-aged healthy adults (Grässler et al., 2021). Therefore, exercise may have cardioprotective properties, thereby reducing cardiovascular risk in this population. Previous research indicates that aerobic training reduces arterial stiffness in normotensive and hypertensive adults (Li et al., 2015). In addition, numerous studies have demonstrated that the cardiac autonomic function of young and early middle-aged healthy adults improves after participating in an aerobic exercise program (Grässler et al., 2021). Resistance exercise is an essential component of a comprehensive wellness program. However, in normotensive and hypertensive adults, previous trials that have measured the effect of resistance training (RT) on arterial stiffness and HRV have yielded variable results (Farinatti et al., 2021; Li et al., 2015), leading to some uncertainty about the effects of RT on arterial stiffness and HRV. The American College of Cardiology/American Heart Association (Arnett et al., 2019) recommends both aerobic and resistance exercise for hypertensive patients, and combined aerobic and RT has demonstrated greater BP reductions than either form of exercise alone (Dos Santos et al., 2014; Ruangthai and Phoemsapthawee, 2019; Son et al., 2017a).
We previously demonstrated that combined aerobic and RT can improve BP and myocardial oxygen demand measurements at rest and during exercise (Phoemsapthawee and Sriton, 2021), as well as HRV in young obese prehypertensive men (Phoemsapthawee et al., 2019). However, research on its impact on arterial stiffness and cardiac autonomic control, which are important factors in the pathophysiology of prehypertension in young people, has been limited. For example, the combination of aerobic and RT either had no effect in rowers (Kawano et al., 2012; Petersen et al., 2006) or improved arterial stiffness in postmenopausal women with and without HT (Figueroa et al., 2011; Son et al., 2017b) as well as in obese prehypertensive adolescent girls (Son et al., 2017a). Previous studies have yielded inconclusive findings, and there are still no conclusive explanations for the underlying mechanisms.
In addition, maximizing long-term adherence requires consideration of an individual’s preferences. Our previous research demonstrated greater exercise adherence and attendance during combined exercise training as compared to aerobic training alone (Ruangthai and Phoemsapthawee, 2019). Eventually, exercise prescriptions should be based on what produces the highest levels of adherence and the greatest long-term benefits. Therefore, the purpose of the present study was to test the hypothesis that combined aerobic and RT would improve BP, arterial stiffness, and cardiac autonomic imbalance in prehypertensive young men, offspring of hypertensive parents.
MATERIALS AND METHODS
Participants
Thirty prehypertensive males aged 18 to 22 years with a parental history of HT were recruited. Participants were classified as prehypertension with systolic BP (SBP) 120 to 139 mmHg or diastolic BP (DBP) 80 to 89 mmHg. All participants were sedentary, defined as having less than 1 hr of regular exercise training per week. Parents’ history of HT was defined as treatment for essential HT (stage 1 or 2) for at least 2 years. Participants were excluded if they were (a) current smokers; (b) taking any medication that might influence cardiometabolism; (c) had musculoskeletal limitations to physical exercise; (d) had any psychiatric problems; or (e) had been on a weight loss diet in the previous 6 months. Participants’ general information was assessed via a self-reported questionnaire. Participants were informed of potential risks and study protocol prior to signing a written informed consent form. The study protocol was approved by the Kasetsart University Research Ethics Committee (COA no. COA64/043).
Study design
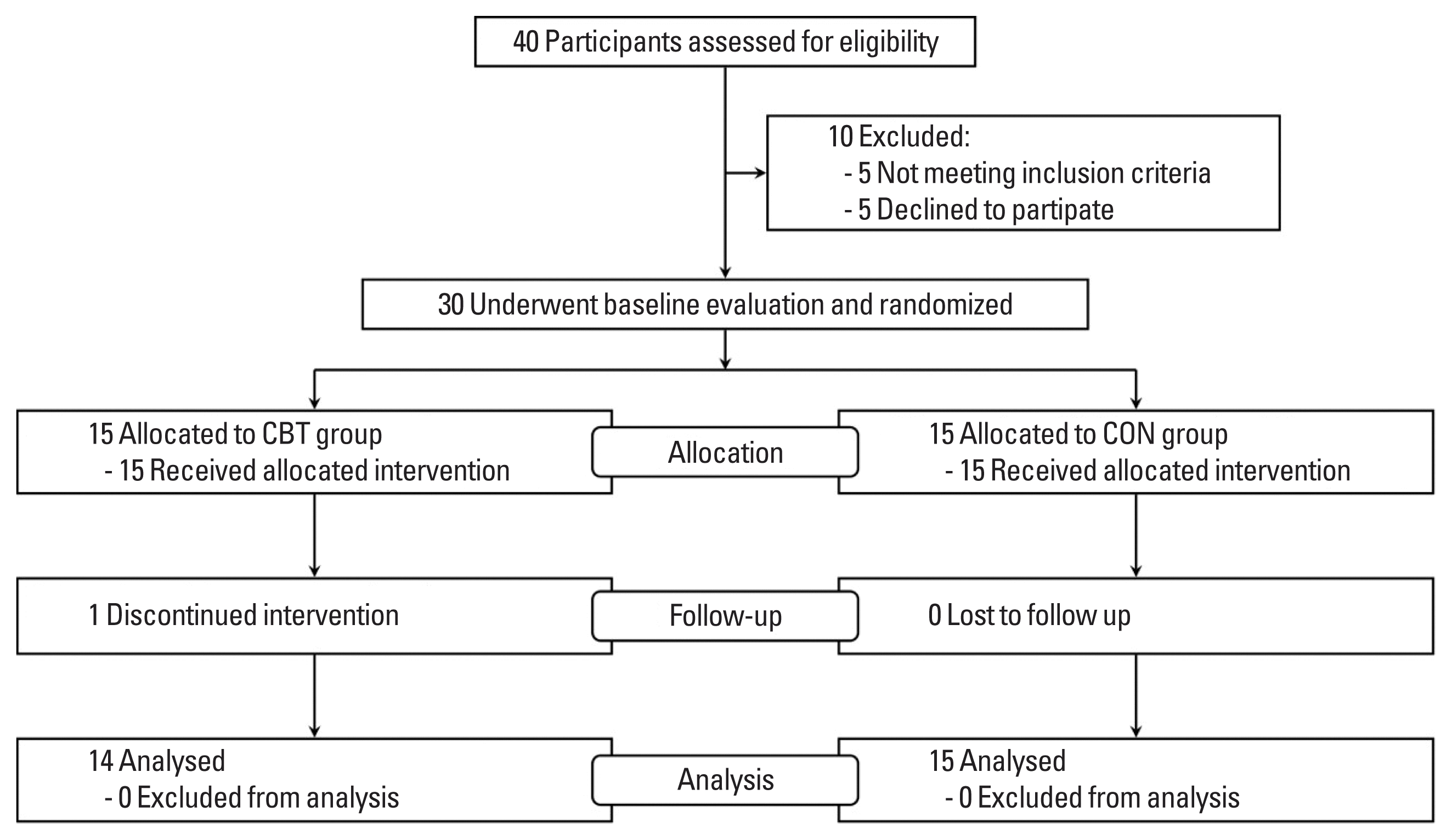
Participants were randomly assigned to a combined exercise training (CBT; n=15) or no exercise group (CON; n=15) using a two-armed, parallel-design (Fig. 1). Prior to and following the 8-week study period, each participant reported to the laboratory in the morning for body composition, BP, arterial stiffness, and HRV assessments. Participants completed a maximal treadmill exercise test using a modified Bruce protocol and a muscular strength test at baseline and after the 8-week period. Participants were reminded once a week to not make any changes to their diet and physical activity habits during the study. They were instructed to maintain their regular diet and exercise routines throughout the duration of the study and were supervised every 2 days by trained coaches (Rathnayake et al., 2014). The participants were supervised throughout the duration of the exercise protocol, but no energy expenditure goal was established. Participants in the CON did not participate in any exercise protocol and were requested to maintain their regular lifestyle (i.e., dietary patterns) for the duration of the study. One participant dropped out of the CBT group because the intervention was discontinued.
CBT program
The CBT program consisted of three 60-min sessions per week for 8 weeks. The exercise session included resistance, aerobic, and stretching exercises: 5 min of dynamic stretching to warm-up, 20 min of resistance exercises, 30 min of treadmill walking, and 5 min of static stretching to cool down. The CBT program was implemented in accordance with our previous study (Phoemsapthawee and Sriton, 2021). To ensure exercise consistency and safety, each training session was supervised by experienced trainers. During the warm-up and cool-down phases, participants performed stretching exercises of the major muscle groups. For the resistance phase, participants performed 12 repetitions per set, 2 sets per exercise, at 50%–70% of one-repetition maximum (1RM) during weeks 1–4. During weeks 5–8, participants performed 8 repetitions per set, 2 sets per exercise, at 60%–80% of 1RM. Each workout included exercises for the upper and lower body: seated rowing machine, bicep curls, shoulder flexions, elbow flexions, push-ups, hip flexions, hip extensions, calf raises, leg presses, and squats. As for the aerobic phase, participants performed 30 min of walking. Exercise intensity was gradually increased from 50%–60% heart rate (HR) reserve in weeks 1–4, to 60%–70% HR reserve in weeks 5–8. The HR of each participant was monitored using a wearable polar device (Polar H7, Polar Inc., Kempele, Finland). The exercise program is shown in Table 1.
Anthropometry and body composition
Height and body weight were taken in the morning after a 12-hr overnight fast. At the same time, body composition was measured. Body weight was measured to the nearest 0.1 kg with a balance beam scale, and % body fat was measured using a bioimpedance analysis device (Inbody 720, Biospace Inc., Seoul, Korea). Body mass index was calculated by dividing body mass (kg) by height squared (m). The waist circumference (W) was measured at the midpoint between the lower rib and the iliac crest at the end of a normal exhalation. The hip circumference (H) was measured around the widest part of the buttocks with the tape parallel to the floor.
Blood pressure
After a 5-min rest, the seated brachial BP of each participant was measured using a standard-calibrated automated brachial sphygmomanometer (Tango M2, SunTech Medical Inc., Morrisville, NC, USA). At a predetermined time in the morning (between 8:00 a.m. and 10:00 a.m.), triplicate measurements were taken consecutively with ≥1 min between each one. The peripheral SBP and DBP were estimated using the average of these three consecutive readings. Mean arterial pressure (MAP) was calculated via the standard equation: MAP =(SBP+2×DBP)/3. Rate-pressure product was calculated by multiplying SBP by HR.
Arterial stiffness
The baPWV, an indicator of arterial stiffness, was measured by a trained technician using an oscillometry-based device (BP-203RPE III; Omron Colin, Co., Tokyo, Japan) in accordance with a standard protocol (Tomiyama and Yamashina, 2010). The oscillometric method has been described and validated previously (Yang et al., 2018). Briefly, each participant rested for at least 5 min and then lay in the supine position with four occlusion and monitoring cuffs wrapped around both ankles and upper arms. Simultaneously, the baPWV, BP, electrocardiogram (ECG), and heart sounds were recorded. Following the collection of bilateral baPWV, the higher one of them was recorded for analysis.
Heart rate variability
The R-R intervals were recorded using a 5-min ECG recording in the sitting position (eMotion Faros device, Mega Electronics, Kuopio, Finland). To avoid possible circadian influences on autonomic function, the ECG was recorded at a fixed time in the morning (between 8:00 a.m. and 10:00 a.m.). Prior to the recording, participants rested comfortably in the supine position for at least 20 min in a temperature-neutral (23°C–25°C) and quiet environment. As determined by visual inspection of chest movement, the respiratory rate was within the normal range (12–20 breaths per minute). The R-R intervals were analyzed using HRV analysis software. Time- and frequency-domain parameters as well as nonlinear HRV components were computed. The time-domain parameters consisted of mean R-R intervals, standard deviation of normal R-R intervals (SDNN), and squared differences between adjacent normal N-N intervals. The frequency-domain parameters consisted of very low-frequency power (VLF power: 0.001–0.04 Hz), low-frequency power (LF power: 0.04–0.15 Hz), and high-frequency power (HF power: 0.15–0.4 Hz). Nonlinear HRV components, Poincaré plot standard deviation perpendicular to the line of identity (standard deviation 1. SD1) and along the line of identity (standard deviation 2. SD2), were analyzed quantitatively by calculating the standard deviations from the R-R interval data.
Cardiorespiratory fitness
Peak oxygen consumption (VO2peak) was determined using a modified Bruce protocol on a treadmill (T-2100 Treadmill, GE Medical Systems, Wauwatosa, WI, USA) in accordance with the guidelines for exercise testing (Fletcher et al., 2001). Expired gas samples were collected on a breath-by-breath mode using a metabolic device (Vmax Encore Metabolic Cart, Vyaire Medical Inc., Yorba Linda, CA, USA), which was calibrated prior to each use according to the manufacturer’s instructions. Throughout the test, oxygen consumption, carbon dioxide production, and HR were continuously recorded and analyzed. At the end of each work rate, a rating of perceived exertion was obtained using the Borg scale (6–20). An automatic sphygmomanometer (Tango M2, SunTech Medical Inc.) was used to measure BP during the final 30 sec of each workload. The average of the three highest consecutive 10-sec averages obtained during the test was used to calculate VO2peak.
Muscle strength
1RM was determined using an indirect method on the leg press, followed by bench presses, as per standard guidelines (Haff and Triplett, 2016). The warm-up consisted of one set of ten repetitions with a light load that participants could complete 12 to 15 times. If the participant performed more than 10 repetitions during these attempts, the load was increased by 30 pounds for the leg press and 10 pounds for the bench press, respectively. The rest period between attempts was 3 min. The workload of 1RM was calculated for each participant based on the loads and repetitions that participants could perform using the 1RM table. Although cadence was not controlled, participants were asked to control the eccentric and concentric movements. All assessments were overseen by two experienced strength-training professionals.
Statistical analysis
Results were expressed as mean±standard deviation. The Shapiro–Wilk test was conducted to examine the normality of the data. An unpaired t-test was used to determine the differences at baseline between groups. A two-way analysis of variance with repeated measures (group [CON and CBT]×time [baseline and after 8 weeks]) was used to determine the effects of combined training and time on dependent variables. If a significant interaction or main effect was noted, univariate analysis was used for post hoc comparisons. An effect size analysis was performed using eta-squared (η2) for the two-way ANOVA, and interpreted 0.01, 0.06, and 0.14 as small, medium, and large, respectively. Correlation among changes (Δ; 8-week value – baseline value) of BP and HRV variables, and baPWV were performed using Pearson correlation. Statistical significance was set at P<0.05. Statistical analyses were conducted using IBM SPSS Statistics ver. 26.0 (IBM Co., Armonk, NY, USA). Based on a previous study (Okamoto et al., 2007), we estimated that 12 men would enable 90% power to detect a 6% decrease in baPWV after a combined training.
RESULTS
The characteristics of participants did not differ significantly between groups. All groups had similar profiles across all clinical parameters examined, as well as a history of HT in the parents (Table 2). Participant adherence to the supervised training program was 100%. Participants completed greater than 98% of the prescribed exercise duration and exercised at 100% of the prescribed exercise intensity during the aerobic training sessions. Participants completed 99% of the prescribed sets and exercised at 100% of the prescribed weight during the RT sessions. Aside from the intervention, levels of habitual physical activity did not change significantly over time, and no difference was observed between groups. There were no significant changes in nutritional intake, including energy, fat, carbohydrates, protein, and sodium.

Participants’ characteristics, body composition, cardiorespiratory fitness, and muscle strength variables at baseline and post the intervention
Body composition
Table 2 shows the body composition parameters in the CON and CBT groups before and after the intervention. There was no significant difference in body mass, body mass index, % body fat, fat mass, fat-free mass, skeletal muscle mass, or waist-to-hip ratio between the CON and CBT groups. Significant group×time interactions were found for waist (η2=0.139, P<0.05) and hip (η2= 0.184, P<0.05) circumferences. post hoc analyses revealed that the CBT regimen resulted in significant waist (P<0.01) and hip (P< 0.01) circumference reductions, but no significant differences between groups.
Cardiorespiratory fitness and muscle strength
There were no significant differences in VO2peak, 1RM bench press, or relative bench press strength between the CON and CBT groups before the intervention. There were, however, statistically significant differences in 1RM leg press and relative leg press strength. There was a significant interaction between the time and the groups in VO2peak (η2=0.242, P<0.05), 1RM leg press (η2=0.366, P<0.01), and relative leg press strength (η2=0.444, P<0.01). In addition, it was found that the 8-week combined exercise intervention exclusively contributed to increasing VO2peak (P<0.05), 1RM leg press (P<0.01), and relative leg press strength (P<0.01), whereas the CON had no statistical time effect. The combined exercise intervention was also effective in increasing VO2peak (P<0.01), 1RM leg press (P<0.01), and relative leg press strength (P<0.01) in the CBT versus the CON. However, no significant interaction existed in 1RM bench press and relative bench press strength.
BP and arterial stiffness
There was no significant difference in baseline values of BP and baPWV between the CON and CBT groups. There were significant group×time interactions for SBP (η2=252, P<0.01), DBP (η2=289, P<0.01), MAP (η2=255, P<0.01), and baPWV (η2= 0.224, P<0.05). post hoc analyses found that the CBT regimen led to significant decreases in SBP (P<0.01), DBP (P<0.01), MAP (P<0.05), and baPWV (P<0.05), but there was no difference in the CON group. Furthermore, the combined exercise intervention was effective in lowering SBP (P<0.05), DBP (P<0.01), MAP (P<0.05), and baPWV (P<0.01) when compared to the CON (Fig. 2).
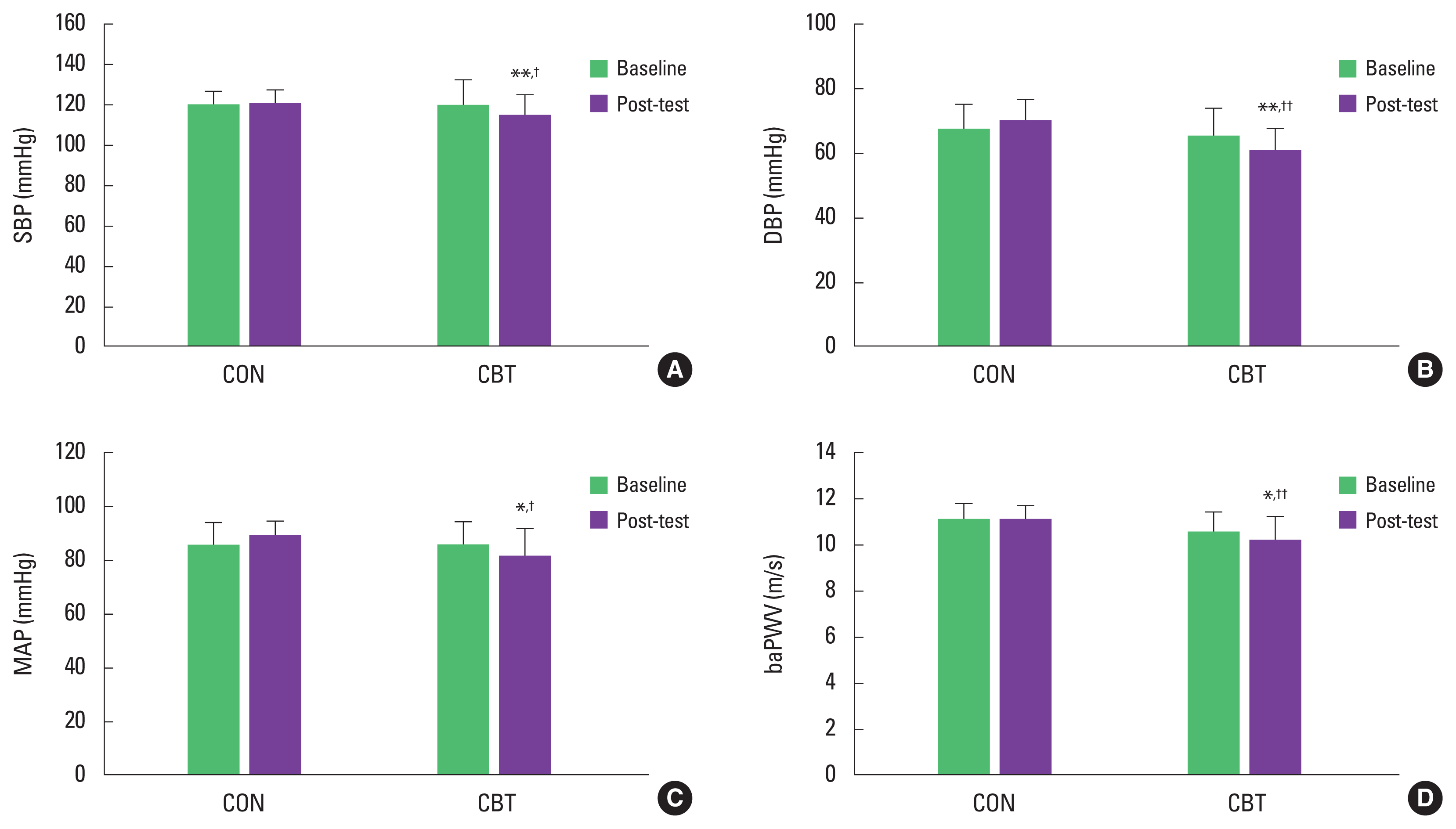
SBP (A), DBP (B), MAP (C), and baPWV (D) at baseline and post the intervention. Data are means±standard deviaiton. CON, control group; CBT, combined exercise training group; SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; baPWV, brachial-ankle pulse wave velocity. *P<0.05 and **P<0.01 to within-group comparison (baseline vs. post the intervention). †P<0.05 and ††P<0.01 to between-group comparison.
Heart rate variability
There were no significant differences between the groups in HRV parameters at baseline. There was a significant interaction between the time and the groups in SDNN (η2=0.223, P=0.01) and VLF (η2=0.147, P<0.05). In addition, it was found that 8-week combined exercise intervention exclusively contributed to increasing SDNN (P<0.01) and VLF (P<0.01), whereas the CON had no statistical time effect. The combined exercise intervention was also effective in increasing SDNN (P<0.05) and VLF (P<0.01) in the CBT versus the CON. However, there was no significant interaction in the other time and frequency domains of HRV (Table 3).
Correlation
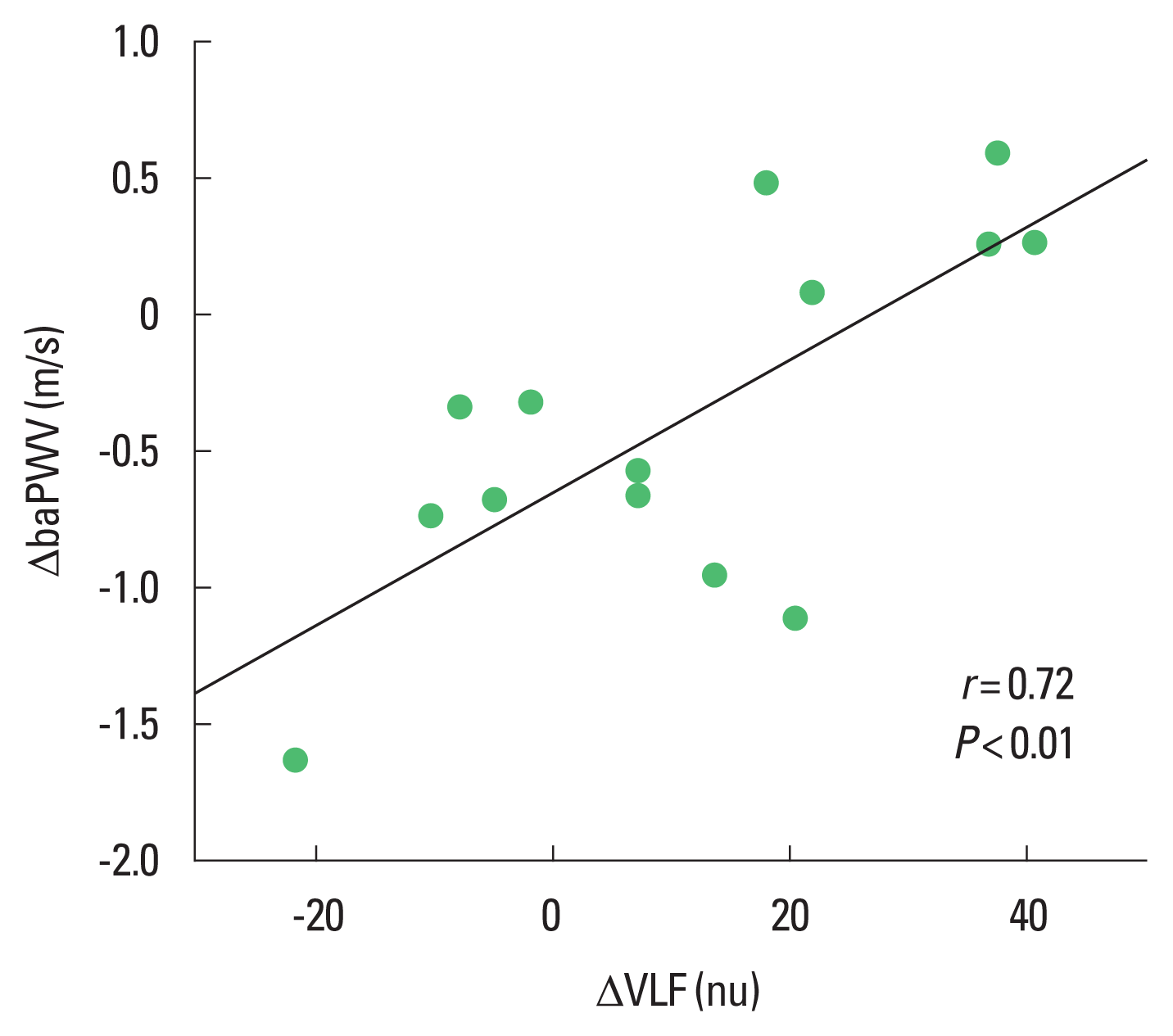
The changes in VLF and baPWV after 8 weeks of CBT were positively correlated (r=0.72, P<0.01) (Fig. 3). There was no significant relationship between VLF and BP changes. Changes in SDNN had no significant correlation with changes in baPWV or BP.
DISCUSSION
The current study reveals a number of novel and interesting findings. The primary finding of the present study was that 8 weeks of CBT improves BP, arterial stiffness, and HRV in prehypertensive young men, the offspring of hypertensive parents. Additionally, a reduction in baPWV was associated with an increase in the VLF power of HRV. This is the first study to our knowledge to demonstrate the positive effects of 8 weeks of CBT on arterial function and cardiac autonomic control in prehypertensive young men with hypertensive parents. These findings suggest that CBT is an effective therapeutic treatment for lowering BP and cardiac autonomic imbalance in this population.
Exercise has been used as a tool in the nonpharmacological treatment or prevention of high BP (Cornelissen and Smart, 2013). Previous studies have demonstrated that 12 weeks of combined resistance and aerobic exercise is both safe and effective in reducing BP and arterial stiffness in obese prehypertensive adolescent girls (Son et al., 2017a) and hypertensive older women (Son et al., 2017b). Furthermore, we previously demonstrated that after 12 weeks of CBT in young obese prehypertensive men, their resting BP and BP during exercise decreased (Phoemsapthawee and Sriton, 2021). We also found that 12 weeks of CBT improved vagal activity in obese young men (Phoemsapthawee et al., 2019). In the present study, we extended the use of this distinct exercise modality to prehypertensive offspring of hypertensive parents and demonstrated that CBT improves SBP (−5.1 mmHg, 3.9% reduction), DBP (−3.9 mmHg, 6.1% reduction), MAP (−4.2 mmHg, 4.7% reduction), arterial stiffness (−0.4 m/sec, 3.5% reduction), and HRV without adverse effects within 8 weeks. In addition, it has been demonstrated that SBP reductions of only 2 mmHg result in significant reductions in the incidence of death due to stroke (7% reduction) and ischemic heart disease or other vascular causes (10% reduction) (Lewington et al., 2002).
High arterial stiffness appears to play a role in the pathogenesis of HT. The offspring of parents with HT had higher arterial stiffness (Andersson et al., 2016). The increased baPWV may be due to increased sympathetic outflow and/or decreased parasympathetic activity, which can lead to additional increases in vascular tone, potentially amplifying the effect of increased peripheral vascular stiffness and BP. We discovered that the 8-week combined exercise intervention exclusively increased SDNN (58.5% increase) and VLF power (34.6% increase). SDNN values represent overall HRV and parasympathetic activity, while VLF power reflects the employment of a parasympathetic efferent limb (Taylor et al., 1998) and renin-angiotensin system activity (Bonaduce et al., 1994). The improvements in baPWV and BP caused by the combined training program could be attributed to increased parasympathetic activity as measured by SDNN and VLF power.
Our findings of lower arterial stiffness and BP after CBT could be explained by increased parasympathetic tone, which is supported by an increase in SDNN and VLF power. We also discovered that lowering baPWV was associated with an increase in the VLF power of HRV, which has been linked to parasympathetic or vasomotor activity. Another possible mechanism responsible for arterial stiffness improvement in this study’s combined training program is an improved endothelium-dependent vasodilator (Son et al., 2017a; Son et al., 2017b), which may be related to reduced oxidative stress and inflammation, as previously reported in our previous study (Ruangthai and Phoemsapthawee, 2019). However, there was no correlation between BP reduction and changes in SDNN or VLF power. The precise mechanism underlying the lower BP is unknown. In addition, his finding warrants further investigation with a larger sample size of subjects.
Low cardiorespiratory fitness (CRF) and muscle strength are significant contributors to increased CVD risk in hypertensive patients (Artero et al., 2012; Kokkinos, 2014). Interestingly, the 8 weeks of CBT increased VO2peak (11.7%) and lower-body muscle strength (30.3%), with a concurrent reduction in baPWV and BP in prehypertensive young men with hypertensive parents. Regarding VO2peak, this suggests that the intensity and duration of the exercise are sufficient to increase CRF. According to our prior research, CBT improves nitric oxide production (Ruangthai and Phoemsapthawee, 2019), resulting in likely improvements in peripheral vasodilation and regional blood distribution (Phoemsapthawee and Sriton, 2021). It could be suggested that increased levels of CRF in prehypertensive offspring of hypertensive parents may contribute to attenuating the progressive increase in arterial stiffness and BP.
As for lower-body muscular strength, since our training program did not increase fat-free mass and skeletal muscle mass, the increases in muscular strength may be the result of neuromuscular adaptations (Brentano et al., 2008). Muscle strength, but not muscle mass, is associated with cardiovascular protection. Previous research found that increased muscle strength was inversely related to arterial stiffness (Fahs et al., 2010) and the prevalence of HT in men (Maslow et al., 2010). These improvements may reduce the risk of adverse cardiovascular events in prehypertensive offspring of hypertensive parents.
The current study has some limitations that should be emphasized. Because the number of subjects studied was small, our findings may be difficult to generalize to other populations. Furthermore, no data in females were available; it is unknown whether the combined exercise would be effective in prehypertensive young women with hypertensive parents.
Our findings indicate that 8 weeks of combined training improves BP, arterial stiffness and vagal-related HRV variables in prehypertension offspring of hypertensive parents. Reduced arterial stiffness is associated with increased parasympathetic activity, which is supported by an increase in VLF power. Additionally, the CBT exercise improves CRF and lower-body muscle strength. These findings suggest that combined training is a useful therapeutic intervention for treating arterial stiffness and high BP and preventing future HT in prehypertensive young men with hypertensive parents.
ACKNOWLEDGMENTS
We wish to thank the participants for their enthusiastic participation throughout the study. The authors received no financial support for this article.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.