Negative neuromuscular and functional repercussion of forced swimming after axonotmesis
Article information
Abstract
Peripheral nerve injuries are cause of sensory disturbances and in functional abilities, and are associated personal and social costs. Strategies that maximize nerve regeneration and functional recovery are necessary, the exercise is an option. This study evaluated the effects of forced swimming exercise on neuromuscular histomorphometry and on functional recovery in a median nerve crush model. Sixteen Wistar rats underwent median nerve crush and were divided into control group (CG) and swimming group (SG). The forced swimming protocol started one week after the injury and was performed for 1 hr a day, 5 days per week, for 2 weeks. The rats swam with an overload of 5% and 10% of body weight in the first and second week, respectively. The functional recovery was assessed in three moments using the grasping test. On day 21, fragments of the median nerve and of the forearm flexors muscles were removed for histomorphometric analysis. The SG had functional recovery impaired (P<0.001) and presented lower myelinated fibers number, fiber and axon minimal diameter, myelin thickness and g-ratio in the proximal e distal segments of the median nerve (P<0.005) and area muscle fiber (P<0.005) than CG. Also, the SG presented a number of capillaries in the proximal segments of the median nerve greater than CG (P<0.005). The exercise protocol used in this study impaired the regeneration of the median nerve and negatively influenced the functional recovery.
INTRODUCTION
Peripheral nerves are common target of injuries, they are affected to varying degrees and when injured become a substantial health problem (Faturi et al., 2016; Gordon, 2016). The peripheral nerve injury promotes motor, autonomic, and sensory alterations in the denervated territory. The peripheral nerve injuries are cause of physical disability, debilitating neuropathic pain, personal and social cost, affecting directly the life quality (Lopes et al., 2022; Maugeri et al., 2021; Panagopoulos et al., 2017; Udina et al., 2011a).
Axons in injured peripheral nerves do regenerate, however recovery of motor function is often limited. Patients’ age, site and degree of injury need to be considered (Faroni et al., 2015; Panagopoulos et al., 2017). The sciatic nerve crush model is the most used in the experimental research on the neuromuscular regeneration and functional recovery after peripheral nerve crush injury (Coradinia et al., 2015; de Moraes et al., 2018; Goulart et al., 2014; Ni et al., 2017; Teodori et al., 2011; Udina et al., 2011b; Yang et al., 2015). However, many peripheral injuries in humans occur in the upper limb which makes the median nerve model clinically relevant (Jager et al., 2014).
The loss of innervation results in atrophy of muscle tissue, which progressively loses its ability to become reinnervated and a rapid impairment on its functional capacity is observed (Borisov et al., 2005; Pedersen, 2011). The muscle preservation or decrease of progressive muscular atrophy is a challenge for patients with peripheral nerve injury (Mandelbaum-Livnat et al., 2016). The motor function recovery is necessary to prevent the denervation-related skeletal muscle atrophy. The time interval between the nerve injury and the restoration of the neuromuscular function is one key factor for the success of this process (Kingham and Terenghi, 2006; Moimas et al., 2013).
In the field of rehabilitation, several resources have been used to accelerate or intensify the axon regeneration and functional recovery (Ni et al., 2017; Santos et al., 2012; Udina et al., 2011a; Udina et al., 2011b). Among them, physical exercise has shown good results on the nerve regeneration, functional recovery and patient’s quality of life postinjury, alone or combined with others axon regeneration promoters (Cintron-Colon et al., 2022; Goulart et al., 2014; Teodori et al., 2011; Udina et al., 2011a; Yang et al., 2015). Trophic factors have been involved in mediating exercise-induced nerve regeneration (Cobianchi et al., 2017; de Moraes et al., 2018; Goulart et al., 2014). Specific exercise modalities to each type of injury can convey more potent neuroprotection and animal studies could help in this regard (Cobianchi et al., 2017).
Swimming is a naturally occurring behavior in rats and is a forced exercise commonly used in the exercise training of animals. The swimming induces limited muscle damage, offers tactile, thermal, and proprioceptive stimuli that can influence the nerve regeneration and attenuates neuropathic pain, favoring functional recovery (Almeida et al., 2015; Teodori et al., 2011; Veskoukis et al., 2018; Yang et al., 2015). There are few studies regarding this exercise modality and neuromuscular regeneration after nerve injury and their results are conflicting, possibly due to type of nerve injury used or differences in exercise protocol – fixed load, water temperature, exercise duration and frequency (Cobianchi et al., 2017; Udina et al., 2011a; Yang et al., 2015).
Considering the exercise as a good option to aid nerve regeneration, the lack of consensus about the most appropriate swimming protocol and the absence of data on the forelimb neuromuscular recovery, the present study aims to investigate the effects of forced swimming exercise on neuromuscular histomorphometry and functional recovery in a median nerve crush model.
MATERIALS AND METHODS
Animals
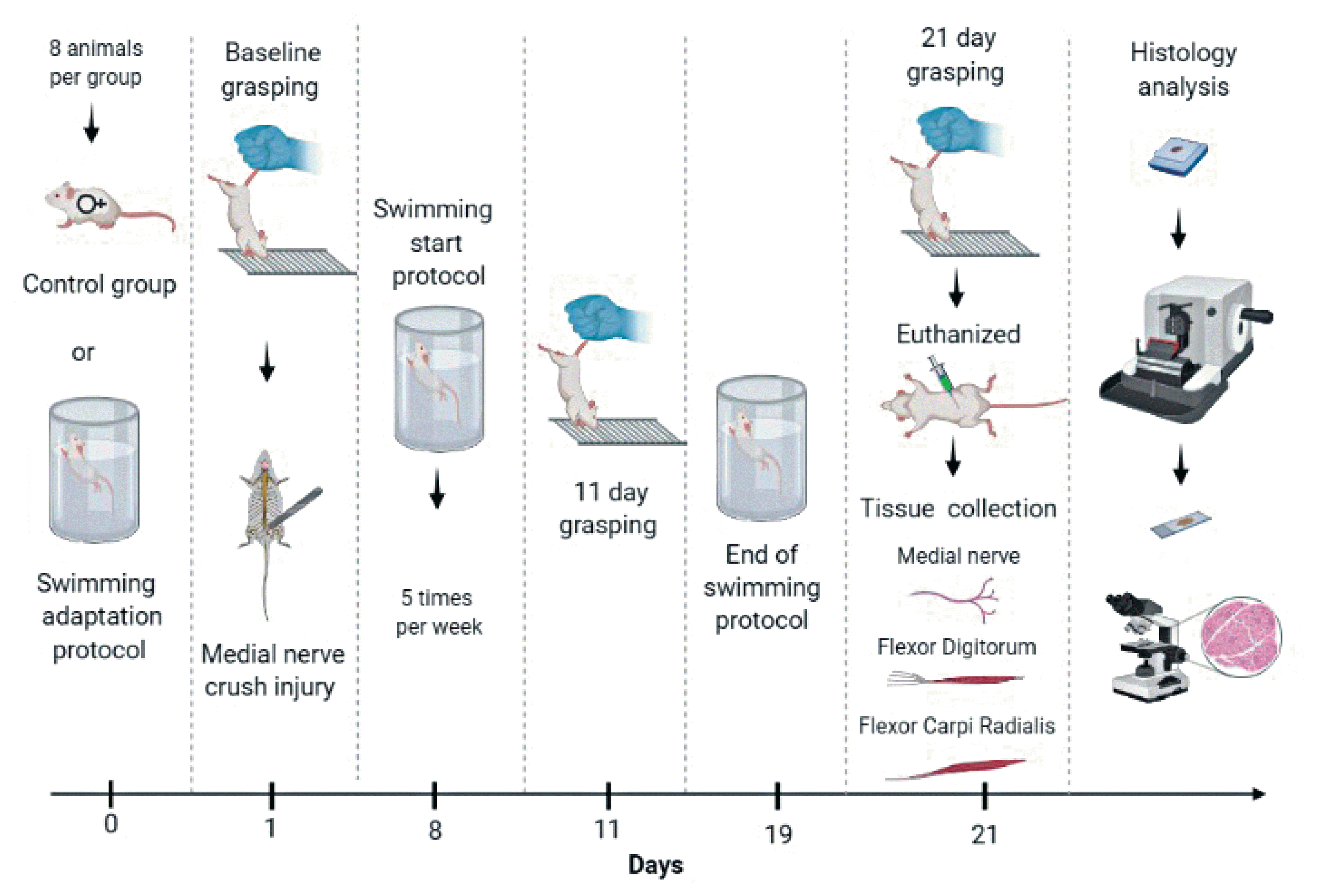
The present study was approved by Animal Ethics Committee (protocol number: 007/12). Sixteen female Wistar rats with 7 weeks old and a mean initial weight of 149±10 g were housed in standard plastic boxes (4 rats per box). They were kept in a constant temperature and humidity environment with a 12-hr light/dark cycle and free access for food (Nuvilab CR1; Nuvital Nutrientes S/A, Paraná, Brazil) and water. The animals were randomly divided into 2 groups: control group (CG) and swimming group (SG). Both groups were submitted to the crush injury of the median nerve, but only the SG underwent to forced swimming program exercises (Fig. 1).
Nerve crush model
For the surgical procedures, the animals were anesthetized with an intraperitoneal injection of xylazine (20 mg/kg) and ketamine (100 mg/kg) (Sespo Ind. Co., Ltd., São Paulo, Brazil). Under aseptic conditions and after hair trimming, the right median nerve was exposed 10 mm above the elbow and a nerve crush injury was induced using a standard hemostat forceps on the second notch maintained during 2 min (Santos et al., 2012). The location of the crush injury was identified with a point on an adjacent muscle. The skin was then closed with 4-0 silk sutures. The animals were placed in their boxes and were kept under supervision until the awake.
Swimming protocol
The animals were placed in a water tank of 85 L capacity (50 cm depth, 85 lengths, and 20 widths) with a water temperature of 31°C. Freestyle swimming was performed 5 days before the surgery for acclimatization, with duration of 10, 20, 30, 45, and 60 min, respectively. The forced swimming protocol started one week after the surgery procedure and was performed for 1 hr a day, 5 days per week, for 2 weeks. The rats swam with an overload of 5% of body weight (BW) attached to tail in the first week and an overload of 10% in the second week. The overload avoided the animals floating behavior. The training was continuously monitored to avoid animal suffering.
Functional evaluation
The recovery of median nerve function was assessed by means of grasping test (Bertelli and Mira, 1995). Briefly, the rats were gently lifted by tail and allowed to grasp a grid connected to an ordinary electronic balance. While grasping the grid, the animal continued to be lifted by the tail with increasing firmness until it lost its grip. At this precise moment, the value showed by the balance was recorded. Before surgery, the function of the right median nerve was assessed in order to obtain baseline control values (presurgery). The animals were then retested on postoperative (day 11) and the end of the experiment (day 21). The contralateral forepaw was temporarily prevented by wrapping it around with an adhesive tape. The tests were made by a single, skilled investigator who was blinded to the experimental group to which each animal belonged.
Nerve and muscle histological and morphometric analysis
Twenty-one days after surgery, the animals were weighted and anaesthetized. The proximal (site of crush) and the distal (forearm, distal 1/3) segments of the median nerve and the flexor digitorum (FD) and flexor carpi radialis (FCR) muscles were removed and fixed in 4% paraformaldehyde solution. The nerves specimens were postfixed with 2% osmium tetroxide. The samples were dehydrated in a graded series of ethanol (70% to 100%) and xylol for diafanization, embedded in Paraplast (Leica, Nussloch, Germany). Cross-sections (5 μm) were made, the muscles were stained with hematoxylin and eosin and the nerves with toluidine blue. The samples were captured and digitized using an optical microscope (Zeiss, Jena, Germany) coupled to an Axio CAM HRc (Zeiss). The muscles were captured in a ×40 magnification; the area and the Feret’s diameter were obtained of 100 muscle fibers randomly selected (Faturi et al., 2016). The luminal area and number of capillaries endoneurial were obtained and 30% of the digitized image of each nerve specimen obtained through the 100x objective was analysed (Santos et al., 2012). Nerve measurements included: number of myelinated fibers, axonal minimal diameter, myelin sheath thickness and the ratio of the axon diameter to the nerve fiber diameter (g-ratio). The ImageJ software (version 1.40, National Institute Health, Bethesda, MD, USA) was used to process the analysis. This process was conducted in a randomized and blind manner.
Statistical analysis
To determine the size sample was used the sample calculation considering an alpha level at 0.05, a test power at 0.80, two-tailed test and known standart deviation (Santos et al., 2012), to detected diffeences between the SG and CG, the appropriate sample size was estimated to be 8 animals. Shapiro–Wilk test was used to determine the distribution (normal or nonnormal) of the data and define the statistical tests. The comparison between groups was performed using Student t-test (for independent samples) and its correspondent nonparametric test (Mann–Whitney test). A repeated measures one-way analysis of variance was performed to compare the three different assessment moments intragroup (presurgery, 11, and 21 days). Post hoc analysis was performed using Tukey test. The significance level adopted for all tests was P≤0.05. The data was expressed as mean±standard deviation. The data was analyzed by using the software GraphPad Prism ver. 5.0 (GraphPad Software Inc., San Diego CA, USA).
RESULTS
Histological and morphometric analysis
The Fig. 2 shows photomicrographs of the proximal (i.e., injury site) and distal segments of the median nerve. Myelinated fibers of larger diameter were observed in the proximal segments of the CG. The endoneurial capillaries were presented sparse in all specimens analyzed, however in larger number in the SG. In the distal segments that difference was less evident.

Digitized images of transverse semithin sections of the regenerated median nerve 21 days after nerve crush lesion. The proximal (A, B) and distal segments (C, D) of the median nerve in the CG (A, C) and SG (B, D) are shown. (A–D) Scale bar is 20 μm. CG, control group; SG, swimming group.
The number of capillaries was greater (P<0.005) in the proximal segments of SG (7.5±2.6) compared to CG (3.5±2.2). There was no difference in the distal segments (CG: 6±3 and SG 4±2). No difference was observed in the area of capillaries when compared proximal and distal segments of the median nerve (CG proximal: 131±67 μm2; SG proximal: 147±74 μm2; CG distal: 53±40 μm2; SG distal: 49±33 μm2). There was no difference in the myelinated fiber number between groups, neither in the proximal nor distal segment. The axonal minimal diameter, myelin sheath thickness and g-ratio were better in the CG. The morphometric data obtained for the proximal and distal segments of the nerve studied are provided in Table 1.
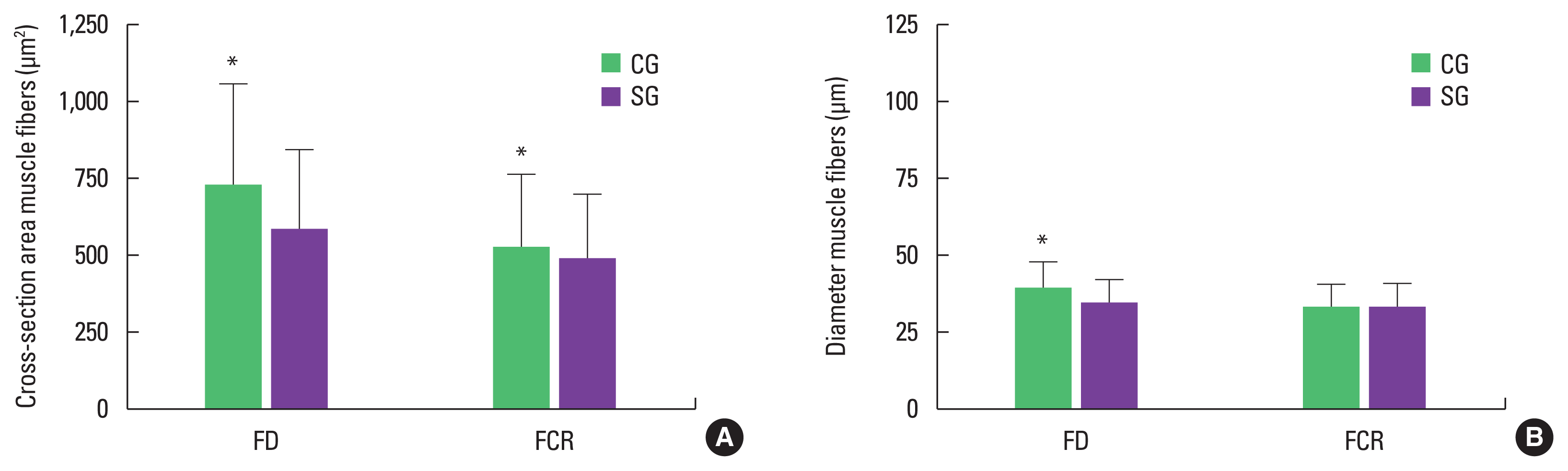
The morphological characteristics of FD and FCR muscle fibers are shown in Fig. 3. The muscle fibers presented polygonal aspect, varied sizes and nucleus located in the periphery, in both groups; although, SG presented reduced muscle fiber size. The morphometric data for muscle fibers is presented in Fig. 4.

Digitized images of transverse semithin sections of the flexor digitorum (A, B) and flexor carpi radialis (C, D) muscles on the 21st day after injury. Control group (A, C) and swimming group (B, D). (A–D) Scale bar is 40 μm. The sections were stained with hematoxylin-eosin.
Functional analysis
The analysis of the data showed significant difference in the three evaluation moments: presurgery, 11° and 21° postoperative day. On the presurgery the SG had better performance (P=0.001) and on the others evaluations the CG had greater performance (P<0.001) showing a better functional recovery (Fig. 5). In the intragroup analyze of the SG the values obtained in 11° and 21° day postoperative were inferior (P=0.001) compared to the presurgery values, whereas the CG presented inferior result only in the 11° (P=0.006).
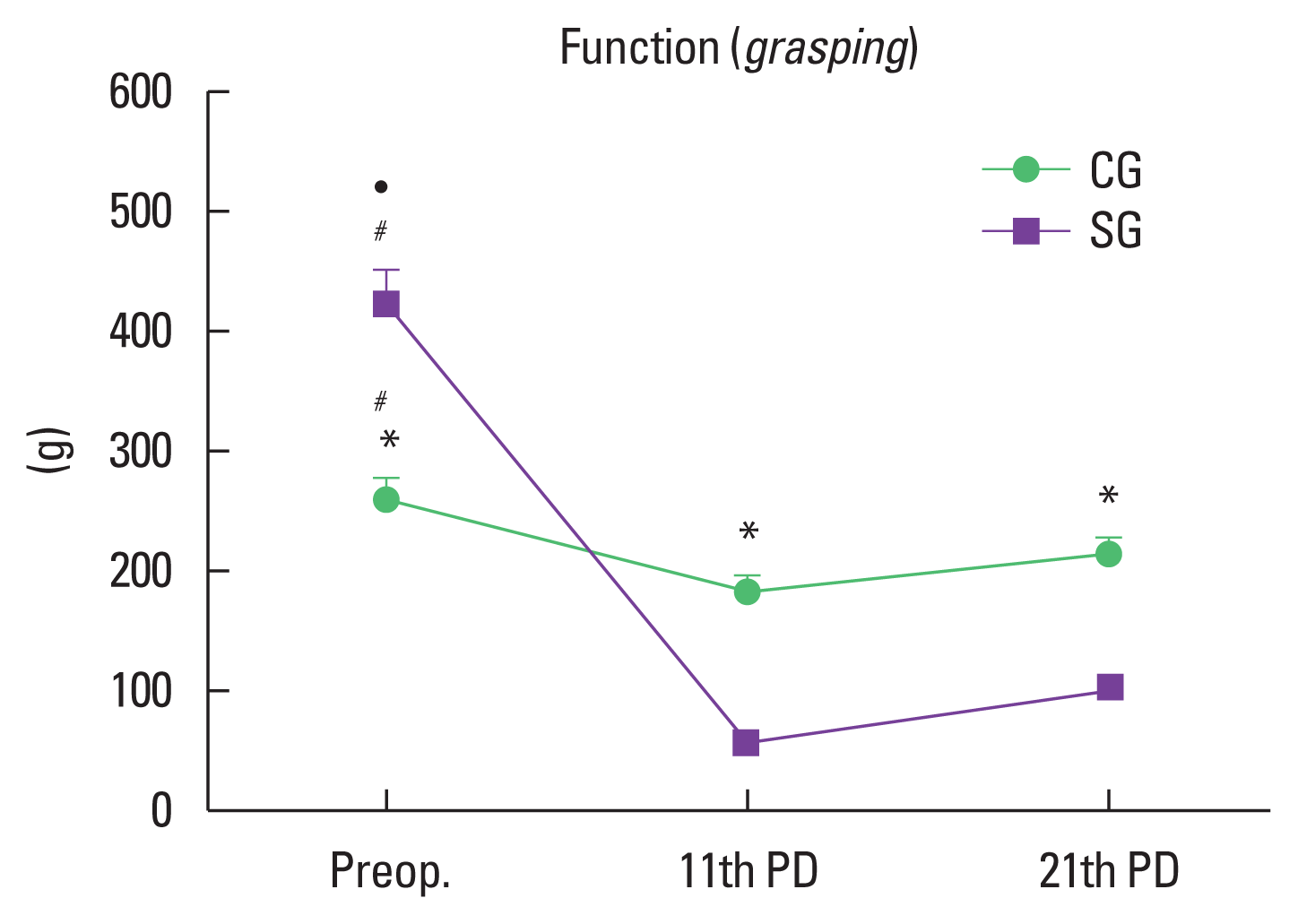
Values obtained by the grasping test. *Significant difference between the groups. #Significant intragroup difference (Preop; 11th PD). •Significant intragroup difference (Preop; 21st PD). Values are presented as the mean±standard deviation. Differences were considered significant at P<0.05. Preop, preoperative day; PD, postoperative day; CG, control group; SG swimming group.
DISCUSSION
The present study investigated the effects of forced swimming exercise on neuromuscular histomorphometry and functional return in a median nerve crush model. The exercise protocol adopted was not favorable for the nerve regeneration. The myelinated fiber diameter, myelin sheath thickness, axonal minimal diameter and g-ratio were smaller on the proximal and distal nerve segments in the SG compared to the CG. Moreover, no functional recovery was found and a reduction on FD and FRC muscles fibers size were observed on the SG.
Despite the negative findings for the exercise protocol proposed, good evolution of the nociceptive threshold and allodynia and for the nerve fiber variables had been shown after swim exercise with overload in sciatic nerve crush model, however the animals swam for 15, 20, and 25 min with an overload of 10% of the BW during 3 weeks (Debastiani et al., 2019), while in the current study the animals swam for an hour with a shorter protocol of 2 weeks. De Moraes et al. (2018) used the swimming as a therapeutic alternative for sciatic nerve regeneration. As well as the current study, the swimming protocol started 7 days after injury. However, the time was gradually added during the 12 first days and on the 3 final weeks it was 15 min without load. Different from the current study, the morphoquantitative results of the nerve were favorable. Furthermore, effects of swimming exercise regimes, 3 times a week from 10 to 30 min without load, on sciatic nerve regeneration in rat, showed improvement in nerve regeneration (Liao et al., 2017).
Results showed a higher number of the endoneurial capillaries on the proximal median nerve segment after forced swimming training. No difference was found for luminal capillaries area. The angiogenic action of exercise is known (Kakihata et al., 2016; Lansford et al., 2016). Similar to that found in this study, Kakihata et al. (2016) reported a larger number of endoneurial blood vessels in sciatic nerve crushed that went through an exercise protocol of aquatic jumps. The assessment of the muscular weight, fibers type and protein concentration indicates that the intense swimming (2 hr day, 5 days week during 3 weeks with load) was not able to improve the reinnervation repair in a denervated muscle after sciatic crush model (Herbison et al., 1974). Swimming exercise stated on the 3rd day after sciatic crush, during 3 weeks and progressive time of 15, 20, and 25 min with an overload of 10% of the BW, did not affect the area of muscle fibers and neuromuscular junctions of the plantar muscle (Santana et al., 2018).
Improvement of motor function recovery after traumatic nerve injury can be obtained by both accelerating axonal regeneration, thus reducing the denervation time, and decelerating the progression of skeletal muscle atrophy (Moimas et al., 2013). The functional recovery was verified through the grasping test, a simple and reliable method for motor function evaluation after median nerve injury (Papalia et al., 2003; Ronchi et al., 2009). Rodents that had the median nerve injured without treatment started the function recovery of the fingers flexor muscles (innervated by the median nerve) on the 8th day postoperative, from the 10th day had already shown good recovery (Bertelli and Mira, 1995) and had fully function restored after the 20th day after surgery (Jager et al., 2014).
In a sciatic crush model, a swimming protocol shorter than the protocol used on the current study (the rats swan during 10 min, for 7 days and it started 3 days after surgery) was beneficial for function recovery (Yang et al., 2015). A study with the same lesion and swimming time (10 min) with overload of 2% to 4%, described an increase on the fibers numbers of the denervated calf muscles (Rhee and Kim, 2013). However, the swimming exercise with overload (10% of the BW) was not beneficial in promoting improvement in grip strength after median nerve crush. The swimming protocol had 3 weeks duration with 20, 30, and 40 min and it started 3 days after injury (Coradinia et al., 2015). Already in an autologous nerve graft model a swimming protocol, 30 min 5 times a week without load did not present differences in both function and motor neuron number (Andrade et al., 2020).
Peripheral nerve injuries represent a substantial clinical problem (Faroni et al., 2015; Lopes et al., 2022; Maugeri et al., 2021), the physical activity increase neuroprotection and improve functional recovery by acting on neurotrophic factors which makes an effective treatment, furthermore it is accessible for everyone (Cobianchi et al., 2017). On the current protocol the association of time (1 hr/day) and overload was not favorable to the nerve regeneration and muscular integrity interfering on the functional recovery. The time chosen may have been a limitation, experimental designs, for example, with 20 min-40 min-60 min protocol or with intervals in longer duration protocols could show favorable results.
Despite the use of specific load (considering the animal weight and its performance) to increase the exercise intensity, the current study was limited once no blood lactate neither the maximal oxygen consumption (maxVO2) were analyzed in order to quantify exactly its intensity. However, the blood lactate is an invasive method and the measurement of the max VO2 in animals during aquatic activities is still a hard procedure. The current study showed negative results after a forced swimming protocol on the nerve and muscle regeneration and functional recovery in rats after an axonotmesis injury. More studies are needed in order to find exercises protocols able to achieve the neuromuscular plasticity desired.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
The authors thank FAPEMIG (Minas Gerais State Agency for Research and Development) for financial support. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.