Exercise with 40-Hz light flicker improves hippocampal insulin signaling in Alzheimer disease mice
Article information
Abstract
We examined whether exercise is associated with hippocampus-mediated improvement in insulin signaling and cell differentiation in the triple transgenic mouse model of Alzheimer disease (3xTg AD) murine model following exposure to 40-Hz light flickering and exercise. We subjected 12-month-old 3xTg AD mice to exercise and 40-Hz light flickering for 3 months. The exercise session was proceeded for 12 consecutive weeks with gradual increase of intensity. To investigate insulin signaling proteins, western blot was conducted to detect the ratio of phosphorylated insulin receptor β (p-IRβ)/total IRβ (t-IRβ), phosphorylated insulin receptor substrate 1 (p-IRS-1)/total IRS-1 (t-IRS-1), phosphorylated phosphatidylinositide-3-kinase (p-PI3K)/total PI3K (t-PI3K), phosphorylated 3-phosphoinositide dependent protein kinase-1 (p-PDK1)/total PDK-1 (t-PDK1), phosphorylated protein kinase B (p-Akt)/total-Akt (t-Akt), and phosphorylated glycogen synthase kinase 3 beta (p-GSK3β)/total GSK3β (t-GSK3β). Doublecortin immunohistochemistry was performed for assessing cell differentiation in the hippocampus. Treatments exerted a positive effect. The combination of exercise and 40-Hz light flickering exposure was the most effective treatment enhancing insulin signaling. Increased ratio of p-IRβ/t-IRβ, p-IRS-1/t-IRS-1, p-PI3K/t-PI3K, p-PDK1/t-PDK1, p-Akt/t-Akt, and p-GSK3β/t-GSK3β and enhanced cell differentiation were observed in the 3xTg AD with exercise under 40-Hz light flickering group. Our results indicate that exercise under 40-Hz light flickering most potently improved insulin signaling, thereby promoted cell differentiation.
INTRODUCTION
Alzheimer disease (AD) is the most prevalent degenerative brain disorder. AD develops dementia over time and deteriorates cognitive abilities, particularly memory. Problems with memory linked to recent events occur in the early stages of AD, along with changes in cognitive function such as language skills and judgment, eventually leading to the full loss of motor function (Kales et al., 2015). Synaptic alteration, structural and functional abnormalities in mitochondria, atypical inflammatory responses, extracellular amyloid-beta (Aβ) plaque deposit, and intracellular neurofibrillary tangles are all linked to AD (Mattson, 2004). Reduction in Aβ clearance or overproduction can cause Aβ accumulation in subcellular compartments, impairs the organelle’s function (Tanzi and Bertram, 2005).
Brain relies on glucose as its primary energy substrates and for sufficient glucose supply to the brain, and insulin signaling plays major role. Impairment of insulin signaling is major feature of type 2 diabetes and it is a risk factor for AD, and these two diseases are linked by several common cellular and molecular processes. Furthermore, these two diseases also exhibit common aspects, such as glucose metabolism dysfunction and insulin resistant (Candeias et al., 2012). Insulin signaling is relevant to memory and cognition modulation, Aβ precursor protein production and clearance, and cerebral glucose metabolism (Wu and Swaab, 2005). Disruption of brain insulin signaling causes the loss of memory functions due to AD (Candeias et al., 2012). The intraneuronal accumulating of neurofibrillary tangles caused by excessive hyperphosphorylation of extracellular deposition of Aβ protein as senile plaques, and widespread neuronal mortality are all major neuropathological features of AD (Yang, 2019).
Since hippocampus, temporal lobe, and cerebral cortex have the highest levels of insulin receptor (IR) expression, these regions are the most susceptible to AD (Moss et al., 1990). In addition, the expression of additional downstream effectors such as phosphatidylinositide-3-kinase (PI3K), protein kinase B (Akt), and glycogen synthase kinase-3 (GSK-3) shows a pattern that resembles that of IR (Leroy and Brion, 1999). The significance of insulin signaling for synaptic function and the existence of active insulin signal transduction in brain areas intimately related with cognition. Furthermore, the hippocampus is one of the initial regions of the brain to be affected by these disorders (Gasparini et al., 2001).
Light therapy has been shown to improve sleep disturbances caused by neurodegenerative disorders such as Parkinson disease (Selkoe, 2001). A study revealed that using a 40-Hz light flicker can stimulate the production of gamma oscillation and reduce the Aβ protein in the brain (Iaccarino et al., 2016). Previous studies have shown that regular exercise is beneficial to the brain’s functioning and can be used in combination with drugs to prevent and treat AD (Adlard et al., 2005). In a study that used familial AD gene mutations, exercise minimized neurotoxicity caused by AD neuropathy. Also, exercise stimulates neuronal regeneration and contributes to an improvement in cognitive functioning through a reduction in beta-secretase activity, decreased accumulation of amyloid plaques and soluble Aβ (Yuede et al., 2009). Therefore, this study investigated the effect of 40-Hz light flickering and exercise on insulin signaling and cell differentiation in the hippocampus of triple transgenic mouse model of Alzheimer disease (3xTg AD).
MATERIALS AND METHODS
Animals
All mice experiments proceeded in accordance with the guidelines of the National Institutes of Health and the Korean Academy of Medical Science. The experimental protocol was approved by the Kyung Hee University Institutional Animal Care and Use Committee (approval number KHUASP [SE]-17-103). The animals were housed under conditions of controlled temperature (24°C±2°C) and lighting (07:00 a.m. to 19:00 p.m.) with food and water ad libitum. 15-month-old male wild type and triple transgenic (3xTg) mice were randomly divided into a wild-type control group, a 3xTg AD group, a 3xTg AD exposed to 40-Hz light flickering group, a 3xTg AD with exercise group, and a 3xTg and exercise under 40-Hz light flickering group (n=10 in each group).
Exercise protocol and 40-Hz light flickering exposure time
The 3xTg mice began exercising at the age of 12 months. The exercise groups exercised on a treadmill once daily in the dark, 6 days per week for 12 consecutive weeks. For adaptation, during the first 3 weeks, mice were given 5 min of warm up at 3 m/min, 30 min of main exercise at 10 m/min, and 5 min of cool down at 3 m/min at 0° incline. Subsequently, mice were subjected to 40 min of the main exercise at 11 m/min for weeks 4 to 6, 50 min of the main exercise at 12 m/min for weeks 7 to 9, and 50 min of the main exercise at 13 m/min for the final weeks 10 to 12. The exposure time of 40-Hz light flickering was consistent with exercise time.
Preparation of tissue
Tissue preparation was proceeded. After the behavior test, the mice were sacrificed. For the brain slices, the mice were anesthetized with ethyl ether, perfused with 50 mM phosphate-buffered saline (PBS), and then fixed with 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections with a thickness of 40 μm were created using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry
Doublecortin (DCX) immunohistochemistry was performed, as the previously described method (Park et al., 2020). Immunohistochemistry was performed for DCX staining in the dentate gyrus to visualize cell differentiation. The sections were incubated in PBS for 10 min and then washed 3 times for 3 min in the PBS. Then incubated the sections in 1% H2O2 for 15 to 30 min. The sections were selected from each brain and incubated overnight with goat anti-DCX antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then with biotinylated rabbit secondary antibody (1:250; Vector Laboratories, Burlingame, CA, USA) for another 90 min. Vector Elite ABC Kit (Vector Laboratories) was used to amplify the secondary antibody. 3,3, diaminobenzidine substrate kit (Vector Laboratories) were used to visualize antibody-biotin-avidin-peroxidase complexes. The slides were air dried overnight at room temperature, and the coverslips were mounted using Permount.
Western blotting
Western blotting was performed, as the previously described method (Kim et al., 2020). Hippocampal tissues were homogenized on ice and lysed in lysis buffer. The protein content was measured using a colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Thirty micrograms of protein were separated on sodium dodecyl sulfate-polyacrylamide gels and transferred on to a nitrocellulose membrane. Then, incubated with mouse β-actin (1:1,000; Santa Cruz Biotechnology), total IR β (t-IRβ) and phosphorylated IRβ (p-IRβ) (1:1,000; Cell Signaling Technology, Danvers, MS, USA), total IR substrate 1 (t-IRS-1) and phosphorylated IRS-1 (p-IRS-1) (1:1,000; Cell Signaling Technology), t-PI3K and p-PI3K (1:1,000; Cell Signaling Technology), total 3-phosphoinositide dependent protein kinase-1 (t-PDK1) and phosphorylated PDK1 (p-PDK1) (1:1,000; Cell Signaling Technology), total-Akt (t-Akt) and phosphorylated Akt (p-Akt) (1:1,000; Cell Signaling Technology), total glycogen synthase kinase 3 beta (t-GSK3β) and phosphorylated GSK3β (p-GSK3β) (1:1,000; Cell Signaling Technology). Horseradish peroxidase-conjugated secondary anti-mouse antibodies were used for β-actin and horseradish peroxidase-conjugated secondary anti-rabbit antibodies were used for t-IRβ, p-IRβ, t-IRS-1, p-IRS-1, t-PDK, p-PDK, t-PI3K, p-PI3K, t-Akt, p-Akt, t-GSK3β, and p-GSK3β.
Statistical analysis
Cell counting and detected bands were quantified using Image-Pro Plus (Media Cyberbetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). The data were analyzed with one-way analysis of variance, followed by the Duncan post hoc tests. All values are expressed as the mean±standard error of the mean, and P-values of <0.05 were considered significant.
RESULTS
Effect of exercise with 40-Hz light flicker on the number of DCX-positive cells in hippocampal dentate gyrus
Fig. 1 shows the number of DCX-positive cells in the hippocampus dentate gyrus. 3xTg AD group showed decreased DCX- positive cell number compared to wild-type control group (P<0.05). In contrast, exercise or 40-Hz light flicker significantly increased the number of DCX-positive cells compared to AD group (P<0.05). In the number of DCX-positive cells, 3xTg AD with exercise under 40-Hz light flickering group showed a significant increase compared to 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group (P<0.05), while there was no significant difference between 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group.
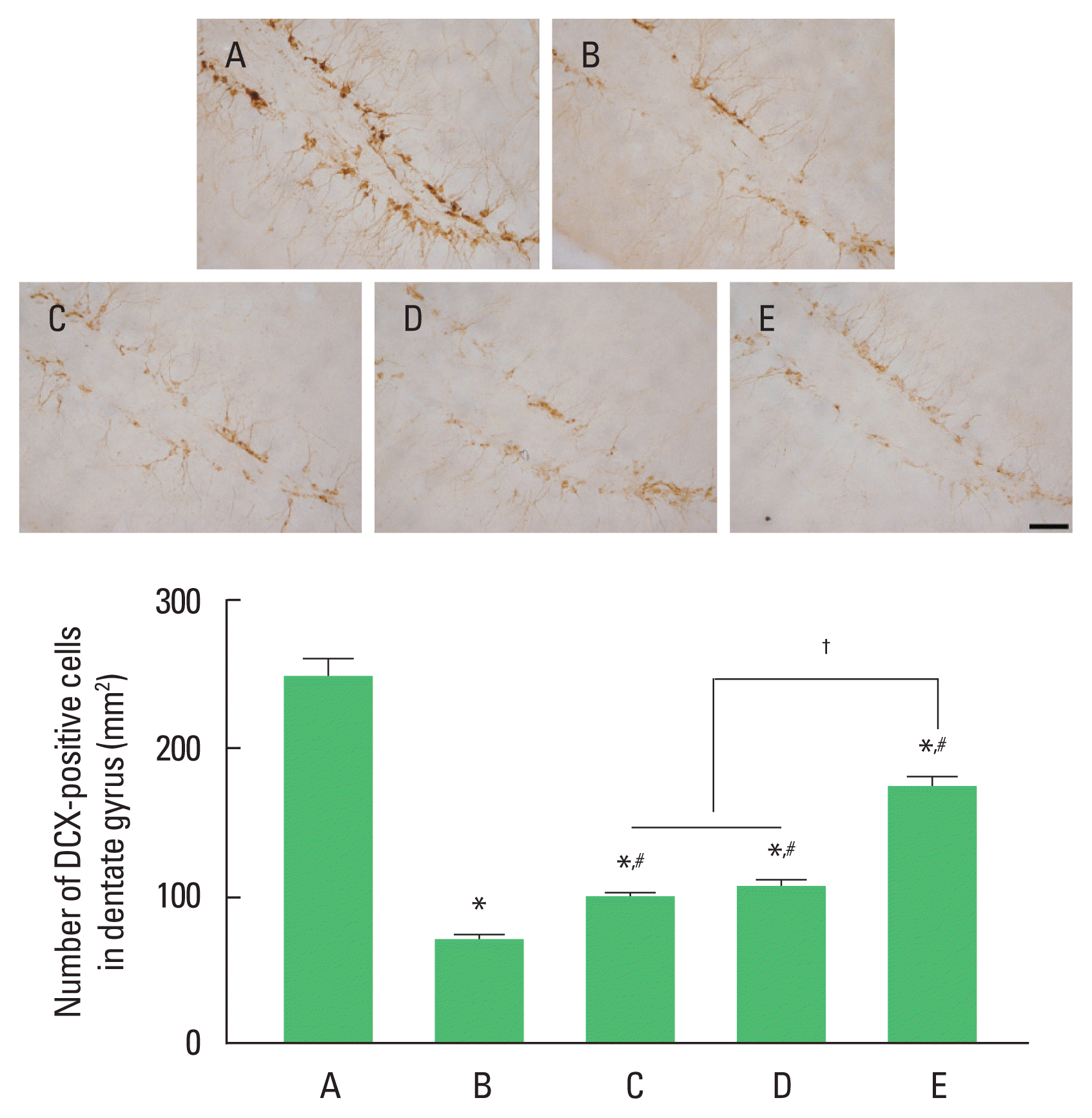
Effect of exercise under exposure to 40-Hz light flicker on cell differentiation in hippocampus dentate gyrus. Upper panel: photomicrographs of doublecortin (DCX)-positive cells. The scale bar represents 50 μm. Lower panel: data of DCX-positive cells in each group. A, wild-type control (CON) group; B, triple transgenic mouse model of Alzheimer disease (AD) group; C, AD exposed to 40-Hz light flickering group; D, AD with exercise group; E, AD with exercise under 40-Hz light flickering group. *P<0.05 compared to CON group. #P<0.05 compared to AD group. †P<0.05.
Effect of exercise with 40-Hz light flicker on the ratio of p-IRβ/t-IRβ and p-IRS-1/t-IRS-1 in hippocampus
For analysis of western blotting data, we set each cascade of the wild-type control group to 1.00. Fig. 2 shows the relative expression of t-IRβ/p-IRβ and t-IRS-1/p-IRS-1 in the hippocampus dentate gyrus. The ratio of p-IRβ/t-IRβ and p-IRS-1/t-IRS-1 were significantly decreased in the AD group compared to those in the wild-type control group (P<0.05). In contrast, exercise or 40-Hz light flicker significantly increased the ratio of p-IRβ/t-IRβ and p-IRS-1/t-IRS-1 compared to AD group (P<0.05). In the ratio of p-IRβ/t-IRβ and p-IRS-1/t-IRS-1, 3xTg AD with exercise under 40-Hz light flickering group showed a significant increase compared to 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group (P<0.05), while there was no significant difference between 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group.
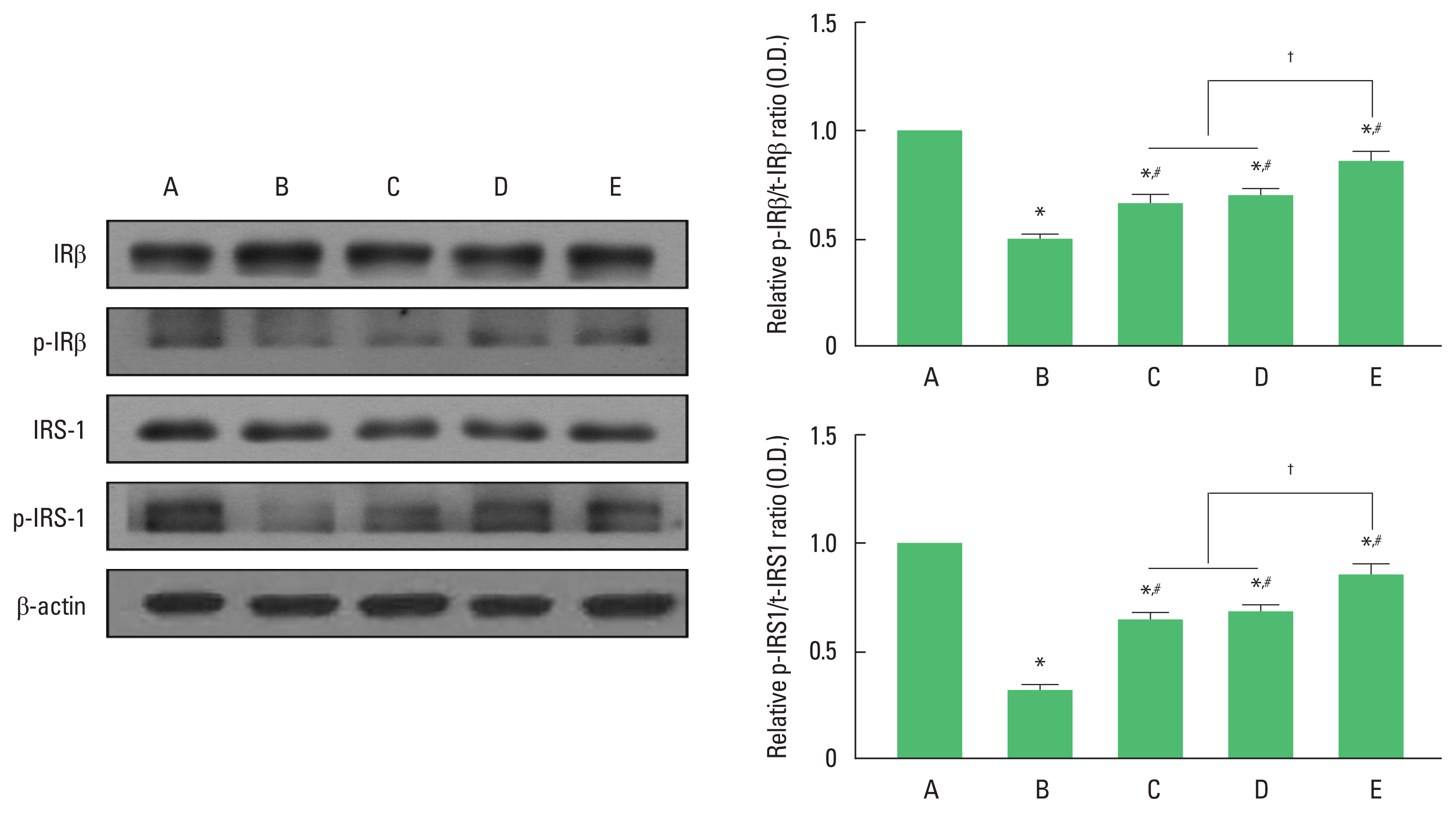
Effect of exercise under exposure to 40-Hz light flicker on the expression of insulin receptor β (IRβ) and insulin receptor substrate 1 (IRS-1). Left panel: representative expression of IRβ and IRS-1. Right upper panel: ratio of phosphorylated IRβ (p-IRβ)/total IRβ (t-IRβ). Right lower panel: ratio of phosphorylated IRS-1 (p-IRS-1)/total IRS-1 (t-IRS-1). A, wild-type control (CON) group; B, triple transgenic mouse model of Alzheimer disease (AD) group; C, AD exposed to 40-Hz light flickering group; D, AD with exercise group; E, AD with exercise under 40-Hz light flickering group. *P<0.05 compared to CON group. #P<0.05 compared to AD group. †P<0.05.
Effect of exercise with 40-Hz light flicker on the ratio of p-PI3K/t-PI3K and p-PDK1/t-PDK1 in hippocampus
Fig. 3 show the relative expression of t-PI3K/p-PI3K and t-PDK1/p-PDK1 in the hippocampus dentate gyrus. The ratio of p-PI3K/t-PI3K and p-PDK1/t-PDK1 were significantly decreased in the AD group compared to those in the wild-type control group (P< 0.05). In contrast, exercise or 40-Hz light flicker significantly increased the ratio of p-PI3K/t-PI3K and p-PDK1/t-PDK1 compared to AD group (P<0.05). In the ratio of p-PI3K/t-PI3K and p-PDK1/t-PDK1, 3xTg AD with exercise under 40-Hz light flickering group showed a significant increase compared to 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group (P<0.05), while there was no significant difference between 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group.
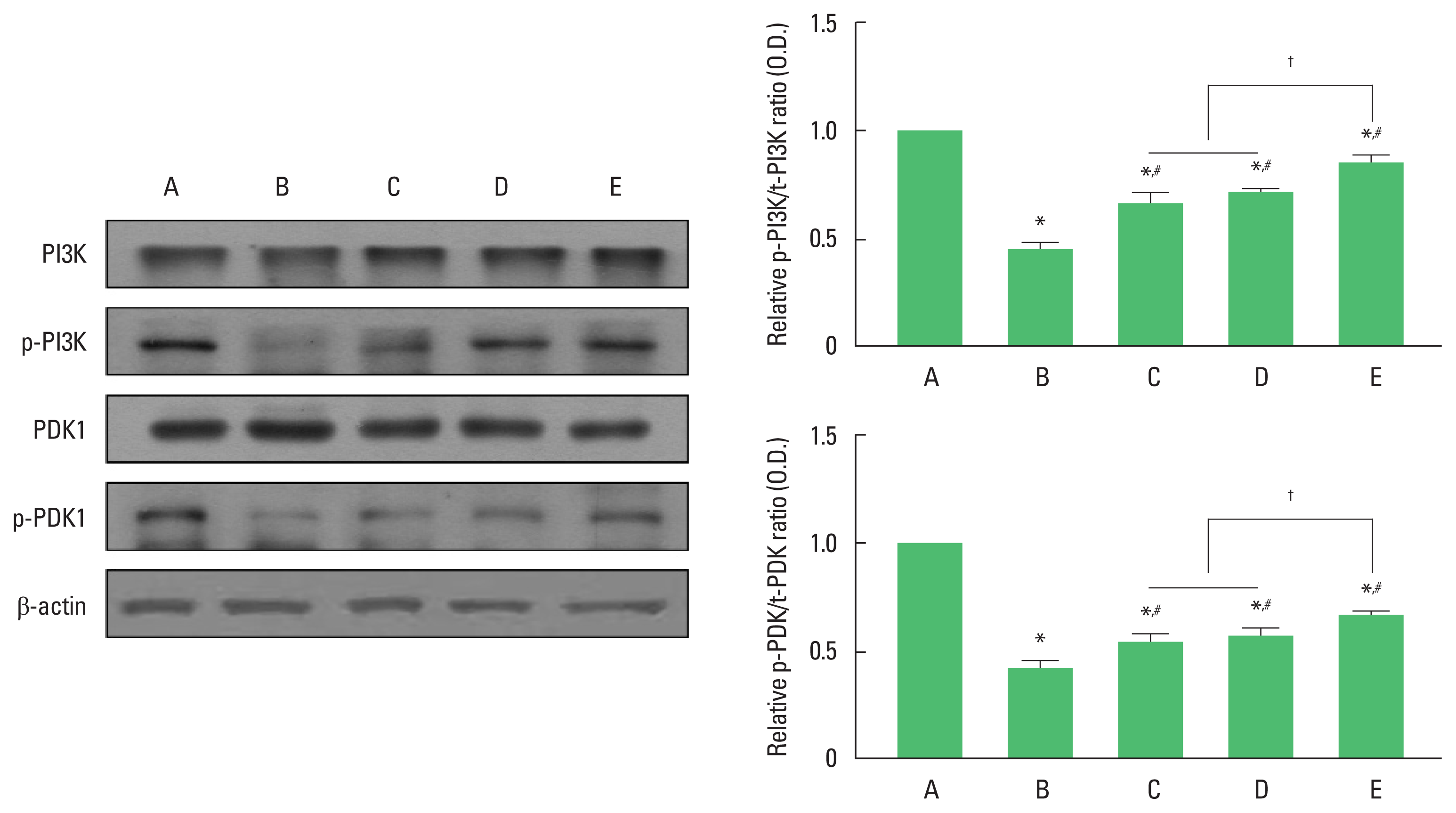
Effect of exercise under exposure to 40-Hz light flicker on phosphatidylinositide-3-kinase (PI3K) and 3-phosphoinositide dependent protein kinase-1 (PDK1). Left panel: representative expression of PI3K and PDK1. Right upper panel: ratio of phosphorylated PI3K (p-PI3K)/total PI3K (t-PI3K). Right lower panel: ratio of phosphorylated PDK1 (p-PDK1)/total PDK1 (t-PDK1). A, wild-type control (CON) group; B, triple transgenic mouse model of Alzheimer disease (AD) group; C, AD exposed to 40-Hz light flickering group; D, AD with exercise group; E, AD with exercise under 40-Hz light flickering group. *P<0.05 compared to CON group. #P<0.05 compared to AD group. †P<0.05.
Effect of exercise with 40-Hz light flicker on the ratio of p-Akt/t-Akt and p-GSK3β/t-GSK3β in hippocampus
Fig. 4 show the relative expression of p-Akt/t-Akt and p-GSK3β/t-GSK-3β in the hippocampus dentate gyrus. The ratio of p-Akt/t-Akt and p-GSK3β/t-GSK3β were significantly decreased in the AD group compared to those in the wild-type control group (P< 0.05). In contrast, exercise or 40-Hz light flicker significantly increased the ratio of p-Akt/t-Akt and p-GSK3β/t-GSK3β compared to AD group (P<0.05). In the ratio of p-Akt/t-Akt and p-GSK3β/t-GSK3β, 3xTg AD with exercise under 40-Hz light flickering group showed a significant increase compared to 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group (P<0.05), while there was no significant difference between 3xTg AD exposed to 40-Hz light flickering group and 3xTg AD with exercise group.
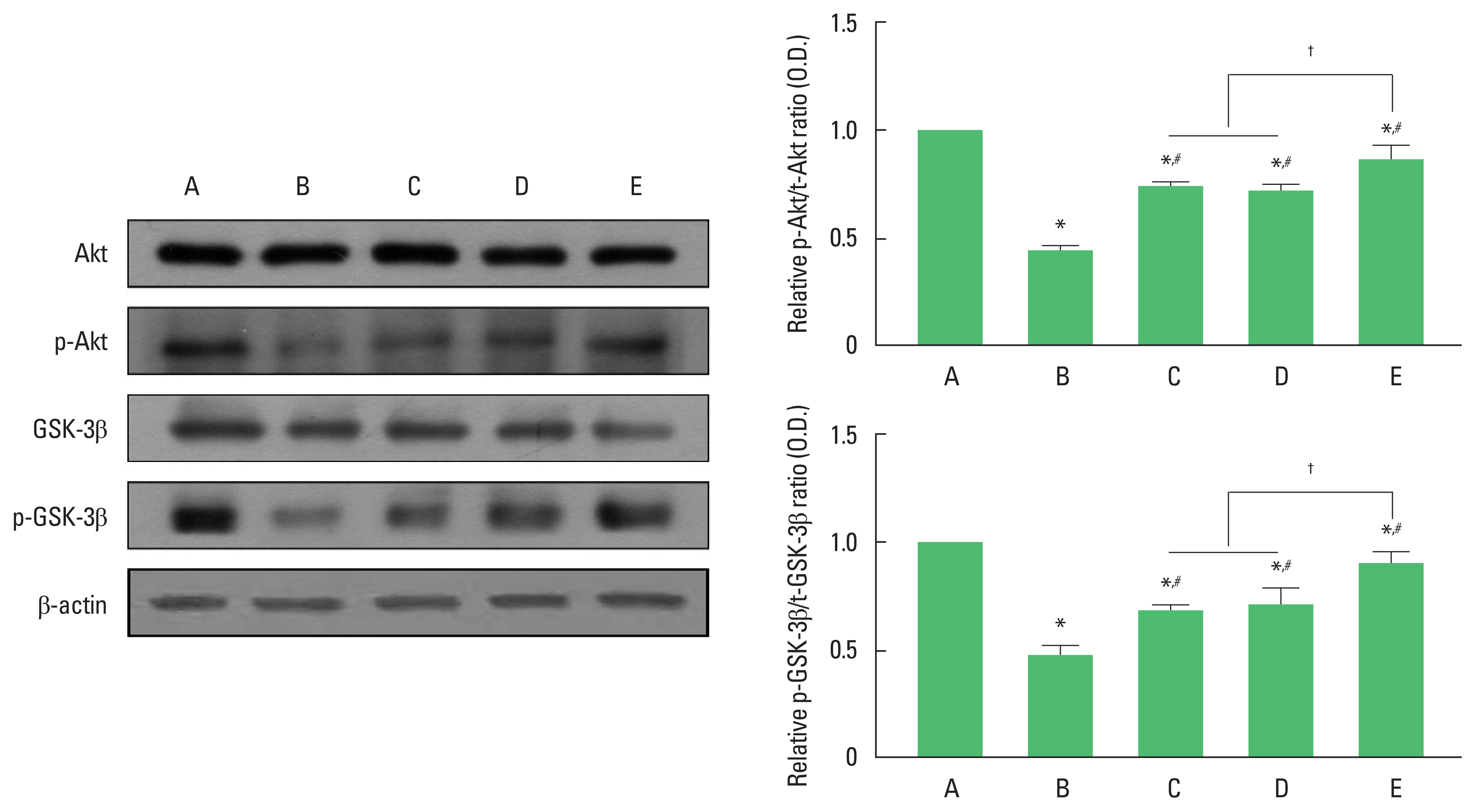
Effect of exercise under exposure to 40-Hz light flicker on protein kinase B (Akt) and glycogen synthase kinase 3 beta (GSK3β). Left panel: representative expression of Akt and GSK3β. Right upper panel: ratio of phosphorylated Akt (p-Akt)/total-Akt (t-Akt). Right lower panel: ratio of phosphorylated GSK3β (p-GSK3β)/total GSK3β (t-GSK3β). A, wild-type control (CON) group; B, triple transgenic mouse model of Alzheimer disease (AD) group; C, AD exposed to 40-Hz light flickering group; D, AD with exercise group; E, AD with exercise under 40-Hz light flickering group. *P<0.05 compared to CON group. #P<0.05 compared to AD group. †P<0.05.
DISCUSSION
Insulin has neuromodulatory effects in various areas of the brain. Irregularity in insulin signaling pathway molecules was considered to have a role in the development of AD (Aleman et al., 2009). Insulin signaling initiates when insulin binds to and activates the IR, which causes additional substrates to be activated and phosphorylated. Aβ has been reported to compete with insulin for IR binding and insulin binding affinity to IR is reduced as a result of this competition, resulting in insulin resistance (Xie et al., 2002). Previous research reported that low IRS-1, PDK1 was seen in AD. PDK1 is a kinase that facilitates in the stimulation of Akt phosphorylation and has been reported to be reduced in AD patients (Cen et al., 2007). Akt is involved in a variety of cellular activities, including metabolism, transcription, protein synthesis, neuron proliferation, growth, and survival. Decreased Akt gene has also been found in AD patients (Chen et al., 2012). GSK-3 is a serine/threonine-specific protein kinase that participates in a variety of cell signaling pathways. GSK-3 has been discovered to be increasingly essential in cellular pathways such as insulin signaling, glycogenesis, neurotrophic factor signaling, neurotransmitter signaling (Ding et al., 2000). GSK-3 is activated by Aβ, causing tau protein phosphorylation, and tau overexpression leads to greater Aβ plaque formation (Hernández et al., 2010). GSK-3 is blocked by GSK3α or GSK3β phosphorylation of Ser 21 or Ser 9 and is enhanced in the lack of Akt activation (Townsend et al., 2007). Previous research has revealed that in the 3xTg AD mice, the expression of p-GSK3β and p-Akt reduced while the expression of Aβ elevated concurrently (Li et al., 2018). In the present study, the activation of insulin signaling pathway including IR and downstream proteins was decreased in 3xTg mice. Increased insulin resistance due to Aβ disturbances downregulated the insulin signaling pathway.
Thus, insulin is an essential trophic element in brain development. Furthermore, the activation of insulin pathway controls neuroblast diffusion from quiescence (Sousa-Nunes et al., 2011). Insulin enhances neurogenesis by regulating neural stem cell proliferation, differentiation (Brooker et al., 2000). In our study, cell differentiation was significantly lower compared to wild-type control group. Because a sufficient supply of glucose cannot be established, the downregulation of insulin signaling in the hippocampus may impact cell differentiation. Furthermore, reduced activation of Akt may affect other cell signaling pathway and result in impairment of metabolism.
The 40-Hz light flicker stimulates the brain and restores gamma rhythms. In a mouse model of AD, 40-Hz light flicker decreased Aβ, resulting in memory improvements (Iaccarino et al., 2016; Singer et al., 2018). 40-Hz light flickering induced gamma oscillations in the visual cortex, hippocampus, and prefrontal cortex, improving spatial learning and memory as well as protein levels of various synaptic signaling and synaptic plasticity markers (Adaikkan et al., 2019). In the present study, AD model exposed to 40-Hz light flicker showed significant enhancement on insulin signaling and cell differentiation compared to AD group. Since Aβ competes with insulin for IR binding (Xie et al., 2002), it is plausible to claim that reduced Aβ by 40-Hz light flicker ameliorates insulin signaling in AD hippocampus. In addition, increased p-GSK3β/t-GSK3β ratio may have mitigate the inhibition of cell differentiation as shown in our data.
Physical exercise has been reported to improve cell proliferation, differentiation, and neurogenesis (Boehme et al., 2011). Those who engage in regular physical activity have lower plasma Aβ and brain amyloid levels (Brown et al., 2013). Thus, exercise has been shown to delay and protect against cognitive impairments by lowering Aβ and activating p-Akt and p-GSK3β (Ser 9) (Larson et al., 2006; Wang et al., 2019). Additionally, a previous study reported that exercise enhances the hippocampal insulin signaling pathway (Muller et al., 2011). In the present study, AD with exercise group showed improvement on insulin signaling and cell differentiation compared to AD group. As with previous research results, exercise appears to lower insulin resistance and reduced Aβ level to enable insulin signaling pathway. In addition, increased glucose metabolism and Akt activation may promotes cell differentiation and proliferation.
Park et al. (2020) reported that exercise under 40-Hz light flickering significantly lowered Aβ in hippocampus, improved neurogenesis, reduced apoptosis and exhibit enhanced cognitive functions compared to each independent treatment in 3xTg AD mice. In this study, AD exercise under 40-Hz light flickering group showed significant improvement in insulin signaling and cell differentiation. These results indicate that exercise combined with 40-Hz light flickering may have synergetic effect on AD by reducing Aβ and normalizing the metabolic pathway including insulin signaling.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2017S1A5B5A02025250).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
