Effect of treadmill exercise combined with bone marrow stromal cell transplantation on atrophy-related signaling pathway in the denervated soleus muscle
Article information
Abstract
The purpose of this study was to investigate whether combination of low-intensity exercise with bone marrow stromal cell (BMSC) transplantation could regulate protein kinas B (Akt)-mammalian target of rapamycin (mTOR) and Wnt3a-β-catenin signaling pathways for prevention of soleus muscle atrophy after sciatic nerve injury (SNI). The experimental rats divided into 5 groups (n=10): normal control group, SNI+sedentary group (SED), SNI+low-intensity treadmill exercise group (TEX), SNI+BMSC transplantation group (BMSC), SNI+TEX+BMSC transplantation group (TEX+BMSC). Sciatic nerve crush injury was applied into the middle of thigh twice for 1 min and 30 sec at interval. Low-intensity treadmill exercise was comprised of walking at a speed of 4 to 8 m/min for 30 min once a day. cultured BMSC at a density of 5×106 in 50-μL phosphate-buffered saline was injected into the distal portion of the injured sciatic nerves. TEX+BMSC group dramatically up-regulated expression levels of growth-associated protein-43 in the injured sciatic nerve at 2 weeks postinjury. Also, although Akt and mTOR signaling pathway significantly increased in TEX and BMSC groups than SED group, TEX+BMSC group showed more potent increment on this signaling in soleus muscle after SNI. Lastly, Wnt3a and the nuclear translocation of β-catenin and nuclear factor-kappa B in soleus were increased by SNI, but TEX+BMSC group significantly downregulated activity of this signaling pathway in the nuclear cell lysate of soleus muscle. Present findings provide new information that combination of low-intensity treadmill exercise might be effective therapeutic approach on restriction of soleus muscle atrophy after peripheral nerve injury.
INTRODUCTION
It has been known that peripheral nerve injury, including the sciatic nerve, adversely affects quality of life due to prolonged neuropathic pain and motor dysfunction (Zhang et al., 2021). The movement of the human body is closely associated with synaptic plasticity in the central and peripheral nervous systems as well as the release of neurotransmitter (acetylcholine) in the neuromuscular junction (NMJ) of the skeletal muscle fibers. Sciatic nerve innervated the skeletal muscles in the lower leg is the largest peripheral nerve in the human body (Sladjana et al., 2008). Therefore, it has been known that traumatic sciatic nerve injury (SNI) resulted in locomotor dysfunction through muscle atrophy in the foot and thigh (Ganguly et al., 2017), and these functional problems are quite limited in their recovery (Irintchev, 2011).
From a molecular biological point of view, SNI-induced muscle atrophy is caused by inhibition of protein kinas B (Akt)-mammalian target of rapamycin (mTOR) signaling pathway and promoting of the Wnt3a-β-catenin cascades (Kurimoto et al., 2015). mTOR has been known as one of the most widely recognized major proteins for regulating muscle hypertrophy (Yoon, 2017), promoting mTOR signaling cascade by activating Akt (Nandagopal and Roux, 2015). Also, Wnt plays a critical role in various development stages ranging from cell survival and apoptosis (Clevers and Nusse, 2012; Kurimoto et al., 2015), which is classified as noncanonical (β-catenin independent) and canonical (β-catenin dependent) signaling pathway (Albanese et al., 2017). Canonical Wnts is a general metabolic regulator to activate the nuclear translocation of β-catenin (Li et al., 2012) and interactively affect nuclear factor-kappa B (NF-κB). Some previous studies reported that inhibition of Wnt3a-regulated nuclear β-catenin accumulation might support activation of acetylcholine receptors (AChR) in NMJ of skeletal muscle (Wang et al., 2008) and NF-κB activated by canonical Wnt pathway might delay development of skeletal myogenesis in patients with muscle disorders (Bakkar and Guttridge, 2010). However, other previous studies suggested conflicting results that voluntary wheel running (Cheng et al., 2013) or treadmill running exercise (Aschenbach et al., 2006) significantly increased canonical Wnt-β-catenin pathway to prevent the muscle atrophy. Thus, therapeutic potential of Wnt signaling pathway in the skeletal muscle and nervous system remain poorly understood and still controversial.
To accelerate axonal regeneration and functional recovery after SNI, many researchers have widely applied cell transplantation techniques using bone marrow stromal cell (BMSC), neural stem cell, fibroblasts (Ma et al., 2014; Zhao et al., 2020), and they suggested that engrafting BMSC was most effective in promoting Schwann cell proliferation and axonal regrowth in the injured sciatic nerve without side effects (Mohammadi et al., 2012; Wang et al., 2020). In addition, forced or voluntary exercise is one of the rehabilitation approaches to delay muscle atrophy and improve functional recovery after SNI (Boeltz et al., 2013). Looking at some previous studies that investigated the effect of aerobic exercise on skeletal muscle morphology in sciatic nerve regeneration, it was represented that both low-intensity forced and voluntary exercises apparently increased myofibril cross-sectional areas and less collagen around soleus muscle fibers after SNI (Bonetti et al., 2017) as well as facilitated hypertrophy in innervated soleus and gastrocnemius muscles after SNI via increase of regenerating motor axons and activation of Akt-mTOR signaling pathway (Jaiswal et al., 2020; Yuan et al., 2020).
With these results presented by previous studies, BMSC transplantation or low-intensity aerobic exercise is a positive effector for improving locomotor functions after SNI. However, experiment studies on combined application of treadmill exercise and BMSC transplantation to examine cellular and molecular mechanisms of muscle atrophy after SNI are still lacking. Therefore, the purpose of this study was to investigate whether combined low-intensity treadmill training with BMSC engraftment could regulate hypertrophy- and atrophy-related signaling pathway in the soleus after SNI.
MATERIALS AND METHODS
Experimental animals
Male Sprague-Dawley rats (7 weeks old) were purchased for this experiment. The use of rats in this study was approved by Ethical committee of Jeju National University (approval number: 2019-0026). And experimental procedures were conducted in accordance with the guidelines for the Care and Use of Laboratory Animals at Jeju National University. The animals in vivo experiment were divided into five groups with randomization method: the normal control group (CON, n=10), SNI+sedentary group (SED, n=10), SNI+low-intensity treadmill exercise group (TEX, n=10), SNI+ BMSC transplantation group (BMSC, n=10), SNI+TEX+BMSC transplantation group (TEX+BMSC, n=10). Animals were maintained at a constant room temperature of 22°C and 60% of humidity under 12/12-hr light-dark cycle. They were accepted to eat commercial rat chow (Samyang Co., Seoul, Korea) and water ad libitum.
BMSC culture
BMSCs were collected from femur and tibia of young rats (4 weeks old), as the extraction technique described by Kim et al. (2018) and Nakano et al. (2013). Cells were cultured in Dulbecco’s modified Eagle’s medium with 20% fetal calf serum. Five to 7 days later, BMSCs adhered to the base on the culture dishes proliferated to a density of ca. 5×106 in one dish.
SNI and BMSC transplantation
All experiment animals used anesthetized with using an animal inhalation narcosis control (Jeungdo Bio & Plant, Seoul, Korea). First, the rats were placed into the chamber with 2%–2.5% concentration of isoflurane for anesthesia and then 1.5%–1.8% concentration for maintenance during SNI. Sciatic nerve crush injury was applied into the middle of thigh twice for 1 min and 30 sec at interval (Seo et al., 2006). Single dose of 5×106 harvested BMSCs in 30 μL phosphate-buffered saline (PBS) was injected into the injury area using a 30-gauge needle. After surgery, anesthetized animals were then placed on a heating pad maintained at 37°C, and then they were put in their cages for resting.
Treadmill exercise
All rats in this experiment had a treadmill exercise adaptation period for 2 weeks. All animals in exercise groups were rested for 2 days after SNI, and started low-intensity treadmill exercise on third postoperation day. Low-intensity exercise on the treadmill device (Jeungdo Bio & Plant) was comprised of walking at a speed of 4 to 8 m/min for 30 min once a day for 2 weeks postinjury.
Western blot analysis
Protein lysates were extracted from the dissected sciatic nerve tissues into triton lysis buffer, and the nucleus and cytoplasm were separated by nuclear extraction buffer and cytosol extraction buffer. Denatured proteins were separated on sodium dodecyl sulphate-polyacrylamide gel and then transferred onto polyvinylidene difluoride membrane on ice at 200 mA for 2 hr. The membranes were blocked with 5% skim milk, 0.1% Tween 20 in tris buffered saline for 30 min at room temperature. Then, the membranes were incubated overnight with primary antibodies at 4°C. Protein (20 μg) was used for Western blot analysis using anti-GAP-43 mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phosphorylated mTOR rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology, Danvers, MA, USA), anti-phosphorylated Akt rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-Wnt3a rabbit monoclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-β-catenin mouse monoclonal antibody (1:1,000, Santa Cruz Biotechnology), anti-NF-κB rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), anti-β-actin mouse monoclonal antibody (1:2,000, Santa Cruz Biotechnology), anti-GAPDH rabbit polyclonal antibody (1:1,000, Cell Signaling Biotechnology), and goat anti-mouse or goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:1,000, GeneTex Inc., Irvine, CA, USA) were used. The blotting proteins were detected by using Westar ECL substrates (Cyanagen, Bologna, Italy). Analysis of protein density was performed using Chemidoc (Bio-Rad, Hercules, CA, USA).
Immunofluorescence staining
For detection of sprouting axons in the injured sciatic nerve, tissues were embedded and frozen at 20°C. Cross sections (20 μm) were cut on a cryostat. Sections were fixed with 4% paraformaldehyde and 4% sucrose in PBS at room temperature for 40 min, permeabilized with 0.5% Nonidet P-40 in PBS, and blocked with 2.5% horse serum and 2.5% bovine serum albumin for 4 hr at room temperature. The sections were incubated with anti-neurofilament-200 (NF-200) rabbit polyclonal antibody (1:700) (Sigma-Aldrich, St. Louis, MO, USA). And then, they were incubated with rhodamine-goat anti-rabbit secondary antibody (1:600) (Molecular Probes, Eugene, OR, USA) for 1h at room temperature. The stained samples were viewed with a fluorescence microscope (Nikon model E-600, Nikon, Kawasaki, Japan), and the images were captured with a digital camera, and analyzed using Adobe Photoshop Software (version CS6, San Jose, CA, USA). The number and regenerating axons were evaluated by using i-Solution software (Image and Microscope Technology, Goleta, CA, USA).
Statistical analysis
All the data is presented as a mean±standard error. Statistical analysis was performed using one-way analysis of variane followed by Duncan post hoc test. The significance level was set at P<0.05. All graphs were performed by using Prism 6 (GraphPad, La Jolla, CA, USA).
RESULTS
Expression levels of GAP-43 in the injured sciatic nerve
To confirm the effect of combined low-intensity exercise with BMSC transplantation on expression levels of GAP-43, specific regeneration marker, in the sciatic nerve at 2 weeks after SNI, we performed biochemical and histological analysis. As shown in Fig. 1A, GAP-43 levels were significantly increased in TEX and TEX+ BMSC groups compared to those in SED group. In immunofluorescence staining at 2 weeks after SNI, the number of NF-200-stained axons was higher in TEX, BMSC, and TEX+BMSC groups than those seen in the SED group (Fig. 1B).
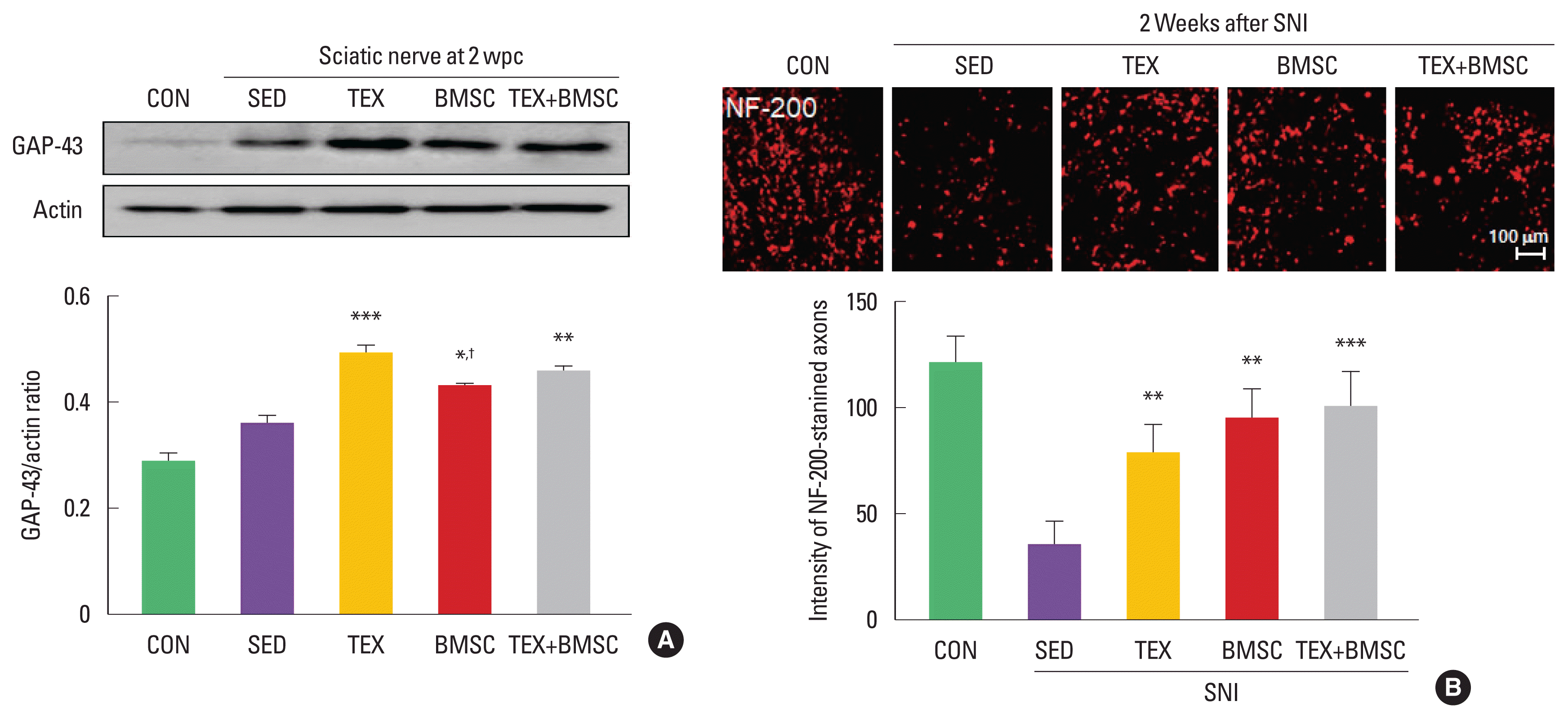
Combination of low-intensity treadmill exercise with bone marrow stromal cell (BMSC) transplantation regulates expression levels of growth-associated protein-43 (GAP-43) and number of neurofilament-200 (NF-200)-stained axons after sciatic nerve injury (SNI). (A) TEX and TEX+BMSC groups significantly increased GAP-43 induction levels in whole cell lysate of the sciatic nerve 2 weeks after SNI. Upper panel: representative expression of GAP-43 protein. Lower panel: the quantification of the ratio of GAP-43 to actin. (B) To investigate regenerating axons in the injured sciatic nerve, immunofluorescence staining was applied into 10 mm distal region to the injury site 2 weeks after SNI. TEX, BMSC, and TEX+BMSC groups showed a significant increase in the number of NF-200-stained regenerating axons when compared to other groups. Upper panel: immunofluorescence staining of NF-200 antibody. Lower panel: the quantification of the intensity of NF-200-positive axons. wpc, week post crush; CON, normal control group; SED, SNI+sedentary group; TEX, SNI+low-intensity treadmill exercise group; BMSC, SNI+BMSC transplantation group; TEX+BMSC, SNI+TEX+BMSC transplantation group. *P<0.05, **P<0.01, ***P<0.001 vs. CON group. †P<0.05 vs. TEX group.
Activation of Akt/mTOR signaling pathway in the soleus after SNI
To examine activation of Akt-mTOR signaling pathway in the soleus at 2 weeks after SNI, we performed Western blot analysis using anti-Akt and mTOR antibodies. As shown in Fig. 2, expression levels of phosphorylated Akt and mTOR were significantly upregulated in TEX or BMSC group compared to those in SED group. In particular, TEX+BMSC group showed more potent increment on phosphorylated Akt and mTOR levels in the soleus compared to TEX group.
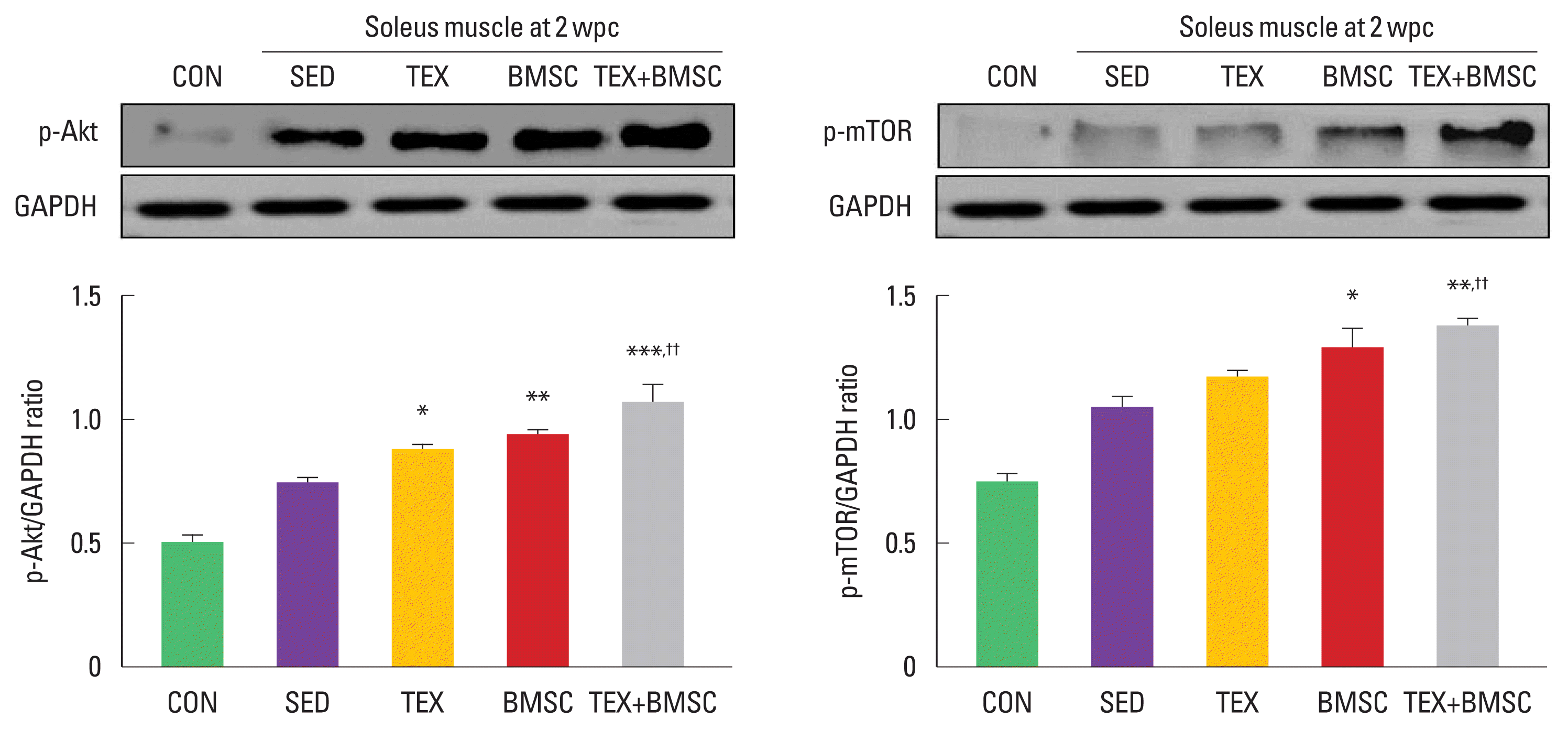
Combination of low-intensity treadmill exercise with bone marrow stromal cell (BMSC) transplantation regulates muscle hypertrophy-related signaling pathway after sciatic nerve injury (SNI). Phosphorylated-protein kinase B (p-Akt) protein was significantly increase in soleus muscle of TEX, BMSC, and TEX+BMSC groups. Phosphorylated-mammalian target of rapamycin (p-mTOR) was further enhanced in BMSC and TEX+BMSC groups than SED group. In particular, both p-Akt and mTOR were dramatically upregulated in TEX+BMSC group. Upper panel: representative expression of p-Akt and mTOR proteins. Lower left panel: the quantification of the ratio of p-Akt to actin. Lower right panel: the quantification of the ratio of p-mTOR to actin. wpc, week post crush; CON, normal control; SED, SNI+sedentary group; TEX, SNI+low-intensity treadmill exercise group; BMSC, SNI+BMSC transplantation group; TEX+BMSC, SNI+TEX+BMSC transplantation group. *P<0.05, **P<0.01, ***P<0.001 vs. CON group. ††P<0.01 vs. TEX group.
Activation of canonical Wnt3a-β-catenin signaling pathway in soleus after SNI
To examine activation of the nuclear translocation of β-catenin in soleus after SNI, we analyzed induction levels of β-catenin from the whole cell or nuclear cell lysate. As shown in Fig. 3, in whole cell lysate, Wnt3a was significantly increased in SED group compared to those in CON group, but TEX group dramatically downregulated Wnt3a levels. In particular, TEX+BMSC group showed more potent decrement on Wnt3a levels in the soleus compared to TEX group. Activation of the nuclear translocation of β-catenin in soleus was significantly decreased in TEX, BMSC, and TEX+ BMSC groups.
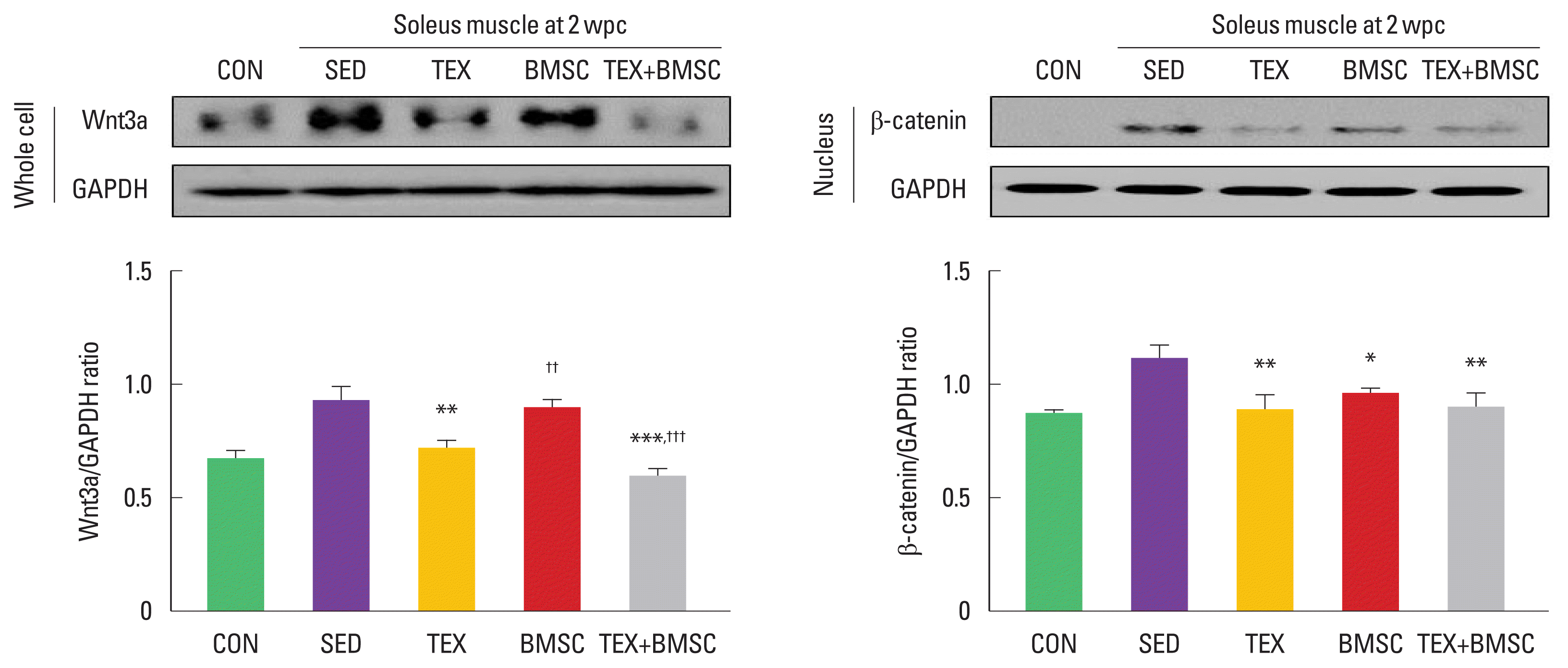
Combination of low-intensity treadmill exercise with bone marrow stromal cell (BMSC) transplantation regulates canonical Wnt3a-β-catenin signaling pathway in soleus muscle after sciatic nerve injury (SNI). In whole cell lysate, Wnt3a was more decreased in soleus of TEX and TEX+BMSC groups than SED group, but applying combination of treadmill exercise and BMSC transplantation sharply downregulated Wnt3a expression levels in soleus compared to TEX group. In nuclear cell lysate, translocation of β-catenin to the nucleus of soleus muscle after SNI was significantly decreased in TEX, BMSC, and TEX+BMSC groups. Upper left panel: representative expression of Wnt3a in whole cell lysate. Upper right panel: representative expression of β-catenin in nuclear cell lysate. Lower left panel: the quantification of the ratio of Wnt3a to actin. Lower right panel: the quantification of the ratio of β-catenin to actin. wpc, week post crush; CON, normal control; SED, SNI+ sedentary group; TEX, SNI+low-intensity treadmill exercise group; BMSC, SNI+BMSC transplantation group; TEX+BMSC, SNI+TEX+BMSC transplantation group. *P<0.05, **P<0.01, ***P<0.001 vs. CON group. ††P<0.01, †††P<0.001 vs. TEX group.
Expression levels of NF-κB in soleus after SNI
NF-κB is a key molecule of inflammation during development and tissue regeneration. To confirm translocation of NF-κB to the nucleus in soleus muscle after SNI, we investigated alterations of NF-κB from the whole cell or nuclear cell lysate. As shown in Fig. 4, in whole cell lysate, NF-κB was significantly enhanced in SED group compared to those in other groups, but TEX, BMSC, and TEX+BMSC groups dramatically downregulated NF-κB levels. But, there were no significant differences of NF-κB expression levels in the cytoplasm between all experimental groups. The nuclear NF-κB accumulation was enhanced in SED group and TEX, BMSC, and TEX+BMSC groups significantly decreased translocation of NF-κB into the nucleus compared to SED group.

Combination of low-intensity treadmill exercise with bone marrow stromal cell (BMSC) transplantation regulates expression of nuclear factor-kappa B (NF-κB), a specific inflammation marker, in soleus muscle after sciatic nerve injury (SNI). In whole cell lysate, NF-κB was more increased in soleus of SED group than CON group, but TEX BMSC and TEX+BMSC groups significantly downregulated NF-κB levels in soleus at 2 weeks postinjury. NF-κB in the nucleus of soleus postinjury was significantly decreased in TEX and BMSC. In particular, TEX+BMSC groups showed the greatest downregulation of NF-κB in both whole and nuclear cell lysates. Upper panel: representative expression of NF-κB protein. Lower left panel: the quantification of the ratio of NF-κB to actin in whole cell lysate. Lower middle panel: the quantification of the ratio of NF-κB to actin in cytoplasm. Lower right panel: the quantification of the ratio of NF-κB to actin in nucleus. wpc, week post crush; CON, normal control; SED, SNI+sedentary group; TEX, SNI+low-intensity treadmill exercise group; BMSC, SNI+BMSC transplantation group; TEX+BMSC, SNI+TEX+ BMSC transplantation group. *P<0.05, **P<0.01, ***P<0.001 vs. CON group.
DISCUSSION
Various previous studies that applied regular exercise and cell transplantation for sciatic nerve regeneration mainly focused on axonal regrowth in the nerves and pain improvement in dorsal root ganglions, but these results do not provide an appropriate mechanism on exercise-induced skeletal muscle changes for functional recovery after SNI. Therefore, we tried to find the effective molecular mechanisms to suppress atrophy of soleus muscle after SNI.
The injured peripheral nerves undergo Wallerian degeneration, which is morphological and biochemical changes in the injured axons. Regeneration process includes Schwann cell proliferation, migration, and differentiation as well as axonal sprouting and remyelination around the injured areas (Caillaud et al., 2019). Among the various regeneration-related molecules, GAP-43 has been well known as a crucial marker secreted from the axon terminal of the injured peripheral nerves (Chung et al., 2020). Jiang et al. (2017) suggested that GAP-43 could promote the axonal growth and remyelination for functional recovery after sciatic nerve transection. Thus, we first confirmed expression levels of GAP-43 in the injured sciatic nerve using Western blot analysis. TEX and TEX+ BMSC groups significantly increased GAP-43 levels compared to SED group, but not in BMSC group. In addition, there are no significant differences TEX and TEX+BMSC groups. In previous studies, GAP-43 started to be expressed from the early phase of the sciatic nerve regeneration and treadmill exercise continuously increased from 1 day to 2 weeks after SNI (Kim et al., 2020; Seo et al., 2009). BMSC-dervied extracellular vesicles treatment also promoted the functional recovery and nerve regeneration after SNI through upregulation of GAP-43 protein (Ma et al., 2017). Although the results in group treated with only BMSC are slightly different from previous data, it is thought that there is a synergistic effect when looking at the findings of combined application of exercise and BMSC.
SNI leads to skeletal muscle atrophy and functional dysfunction (Bonetti et al., 2017). Atrophy of the skeletal muscle is highly associated with a specific mechanism such as phosphorylation of mTOR and canonical Wnt signaling pathway (Bodine et al., 2001; Kurimoto et al., 2015; Nandagopal and Roux, 2015). mTOR regulates protein synthesis and muscle growth, and it is stimulated by combining resistance exercise and appropriate nutritional strategies (Deldicque et al., 2005). Insulin-like growth factor 1, PtdIns-3-OH kinase, and PtdIns-regulated kinase Akt led to activation of mTOR, whose downstream molecules including P70s6k (protein 70 kD ribosomal protein S6 kinase) and 4E-BP1 (eukaryotic translation initiation factor 4E binding protein 1) have promoted protein synthesis and muscle hypertrophy through increases in the initiation and elongation phases of translation (Bodine et al., 2001; Nandagopal and Roux, 2015). Unlike noncanonical Wnt pathway, activation of canonical Wnt signaling pathway accumulate and translocate β-catenin into the nucleus for reducing the sensitivity of AChR in NMJ and skeletal muscle mass (Albanese et al., 2017; Zhang and Peng, 2011). In present study, TEX+BMSC group showed the greatest increase in the activation of Akt-mTOR signaling pathway as well as the greatest decrease in translocation of β-catenin into the nucleus of soleus muscle after SNI. Previous studies demonstrated that activation of Akt/mTOR pathway could increase Schwann cell proliferation and decrease skeletal muscle atrophy after peripheral nerve injury (Zhu et al., 2019) and physical immobilization (Bodine et al., 2001), respectively. Although canonical Wnt/β-catenin signaling pathway is controversial in the remodeling of damaged aged skeletal muscles, many studies have reported that this pathway is generally activated during muscle atrophy and suppressed by physical exercise (Hu et al., 2019; Zhang et al., 2021). These data implicate that combination of low-intensity exercise with BMSC transplantation might be a new therapeutic strategy for improving skeletal muscle atrophy after SNI.
In addition to elucidating the role of canonical Wnt/β-catenin signaling pathway in muscle atrophy, we identified that TEX+ BMSC group sharply downregulated NF-κB levels in soleus at 2 weeks postinjury compared to those in other groups. Skeletal muscle atrophy can result from enhanced protein degradation and downregulated protein synthesis in immobilization (Bar-Shai et al., 2005), aging (Tilstra et al., 2011), and chronic inflammatory diseases (Li et al., 2008) through activation of NF-κB. In disuse muscle atrophy such as sciatic denervation, NF-κB can regulate expression of myostatin, p38, and calcineurin to induce the loss of skeletal muscle mass (Zhang et al., 2007), and this signaling pathway reciprocally influences the activity of canonical Wnt/β-catenin signaling pathway (Ma and Hottiger, 2016). Our results regarding NF-κB secretion blocked by exercise was consistent with some previous showing that NF-κB and Wnt pathways shows multiple cross-talks during skeletal muscle atrophy (Ma and Hottiger, 2016).
Given these results obtained in present study, low-intensity treadmill exercise and BMSC transplantation approaches would positively regulate biochemical mechanisms in nerve and soleus muscle during sciatic nerve regeneration. In conclusion, our findings suggest that combination of low-intensity treadmill exercise with BMSC transplantation should provide may be effective therapeutic approach to restrict soleus muscle atrophy after peripheral nerve injury.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2019R1F1A1062392).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
