Swimming exercise ameliorates mood disorder and memory impairment by enhancing neurogenesis, serotonin expression, and inhibiting apoptosis in social isolation rats during adolescence
Article information
Abstract
Social isolation during adolescence is associated with anxiety, depres-sion, and memory impairment. Exercise has been reported as a positive effect on brain function, especially hippocampus. The present study ex-amined the effect of swimming exercise on apoptosis, cell proliferation, and serotonin expression in social isolation rats during adolescence stage. Social isolation started at postnatal day 21 and continued for 6 weeks. The rats in the swimming group were forced to swim for 60 min once daily during 6 days per week for 6 consecutive weeks. The rats in the social isolation during adolescence showed anxiety, depression, short-term memory impairment. Social isolation facilitated apoptosis and inhibited cell proliferation and differentiation. Social isolation sup-pressed expression of serotonin, brain-derived neurotrophic factor, and tyrosine kinase B. Swimming exercise alleviated anxiety, depression, short-term impairment. Swimming exercise suppressed apoptosis, en-hanced neurogenesis, and increased serotonin expression. In our study, swimming exercise ameliorates mood disorder and memory impairment by enhancing neurogenesis and serotonin expression and inhibiting apoptosis in social isolation.
INTRODUCTION
Social isolation during adolescence causes depressive symptoms and reduces memory function (Dunphy-Doherty et al., 2018). Social isolation at a young age inhibits dendritic development in the hippocampus and induces neurological dysfunction in the limbic system (Silva-Gómez et al., 2003). The hippocampus is an important region of the brain involved in the process of learning and memory formation, and hippocampal neurogenesis continues to take place after the development of the nervous system (López-Toledano and Shelanski, 2004).
Apoptosis is a form of cell death that serves to remove dying cells from cell proliferating or differentiating, thus apoptosis plays a critical role in normal development and tissue homeostasis. Inappropriate or excessive apoptosis, however, is implicated in diverse neurological disorders (Lee et al., 2003). Terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) assay detects DNA fragmentation, one of the character-istics of apoptotic cell death (Gavrieli et al., 1992). Bcl-2 inhibits apoptosis while Bax promotes apoptosis (Song et al., 2018).
Brain-derived neurotrophic factor (BDNF) and its receptor, tyrosine kinase B (TrkB), are abundantly expressed in the mammalian brain, especially hippocampus. Learning and exercise increase hippocampal BDNF expression (Cho et al., 2017; Hall et al., 2000). Exercise-induced BDNF expression facilitates neurogenesis and enhances long-term potentiation of the hippocampus (Farmer et al., 2004).
Immunohistochemistry detects 5-bromo-2′-deoxyuridine (BrdU), and BrdU-positive cells represent newly formed cells (Park et al., 2019). Doublecortin (DCX) is a marker of neuronal precursor cells, and DCX is implicated in neuronal differentiation (Park et al., 2019).
Serotonin (5-hydroxytryptamine, 5-HT) is related with depression or anxiety (Wang et al., 2019). Tryptophan hydroxylase (TPH) is a speed-limiting enzyme for the production of 5-HT and TPH level is an indicator of 5-HT synthesis. Stress from social isolation leads to depression- and anxiety-related behaviors as well as neuroendocrine and physiologic dysfunctions that affect dopamine and 5-HT (McNeal et al., 2018).
Previous studies demonstrated that physical exercise affects therapeutic effects on anxiety, depression, and stress (Ji et al., 2017; Park et al., 2017). The present study examined the effect of swimming exercise on apoptosis, cell proliferation, and serotonin expression in the adolescence rats.
MATERIALS AND METHODS
Experiment animals
This study was approved by the Kyung Hee University Institutional Animal Care and Use Committee (Seoul, Korea) (KHUASP [SE]-16-154). Male Wistar rats were randomly divided into 4 groups (n=8 per group): group housed (GH), group housed and exercise (GH+E), socially isolated (SI), and socially isolated and exercise (SI+E). Social isolation was performed as described by Amiri et al. (2015). At postnatal day 21, the rats were randomly housed for 6 weeks under two different conditions: GH and SI. GH rats were housed (4 per cage) in Plexiglas boxes and SI rats were housed individually in Plexiglas boxes.
Swimming exercise protocol
The rats in the swimming group were forced to swim for 60 min once daily during 6 days per week for 6 consecutive weeks. In swimming pool (120 cm×80 cm×50 cm), filled with water at a temperature of 30°C±2°C. Swimming was performed weighing 5% of the rat body weight.
Elevated plus-maze test
Anxiety-like behavior was evaluated using the elevated plus-maze test as previous method (Park et al., 2019). The elevated plus-maze apparatus consisted of two open arms (45 cm×10 cm), crossed at right two opposed arms of the same size. The junction area of central measured platform (10 cm×10 cm), set up 65 cm above the floor. The rats were placed on the central platform facing a closed arm and were allowed to explore the maze freely for 5 min. Entry into an arm was defined as entry of all four paws into the arm. Times and entries in the open arms were measured.
Forced swimming test
The forced swimming test was used to confirm a depressive state as previous method (Lee et al., 2019). The experimental animals were placed individually in glass cylinders (height, 50 cm; diameter, 15 cm) containing water at a temperature of 25°C and a depth of approximately 30 cm. All rats underwent a 15-min pretest to confirm their adaptation to water. One day after the pretest, the animals were tested for 5 min. During the test session, immobility time was measured using the smart version 2.5 video tracking system. Immobility was defined to occur when no additional activity was observed other than the actions needed by the animal to keep its head above the water.
Step-through avoidance test
In order to evaluate short-term term memory, step-through avoidance test was performed at 24 hr after training as previous method (Park et al., 2019). The latency time (sec) in each group was measured. In the training session, the rat was placed in illuminated compartment, and then guillotine door was raised to allow the rat to enter the dark compartment. When the rat’s hind leg entered the dark compartment, the guillotine door was closed. Electrical foot shock (0.2 mA) was delivered through the grid floor for 2 sec. At 24 hr after training session, this process performed again. The time that elapsed prior to entry into dark compartment was recorded. A latency time over 180 sec was counted as 180 sec.
Tissue preparation
The animals were sacrificed immediately after determination of step-through avoidance test. To prepare the brain slices, the animals were fully anesthetized with ethyl ether, and then the rats were transcardially perfused with 50 mM phosphate-buffered saline. The rats were fixed with freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections with thickness of 40 μm were made using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry for TPH, 5-HT in dorsal raphe
To visualize TPH and 5-HT expression, immunohistochemistry for TPH and 5-HT in the dorsal raphe nuclei was performed as previously described method (Park et al., 2019). The sections were incubated in phosphate-buffered saline (PBS) for 10 min, and then washed 3 times in the same buffer. The sections were then incubated in 1% hydrogen peroxide for 30 min. The sections were incubated overnight with rabbit anti-TPH antibody (1:1,000; Oncogene Research Product, Cambridge, UK), rabbit anti-5-HT antibody (1:500; Abcam, Cambridge, UK) and goat anti-DCX antibody (1:1,000; Oncogene Research Product) and then with biotinylated rabbit and goat secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for another 1 hr. The secondary antibody was amplified with the Vector Elite ABC kit (1:100; Vector Laboratories). Antibody-biotin-avidin-peroxidase complexes were visualized using 0.03% diaminobenzidine (DAB), and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Immunohistochemistry for BrdU
Immunohistochemistry for BrdU was performed as previously described method (Park et al., 2019). BrdU (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) was given intraperitoneally to all animals 1 hr before starting treadmill exercise for the 3 consecutive days prior to sacrifice. The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2X standard saline citrate at 65°C for 2 hr, denatured in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH, 8.5). The sections were then incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). They were then washed three times with PBS and incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories) for 1 hr. The sections were then incubated for 1 hr with an ABC complex (1:100; Vector Laboratories). For visualization, sections were incubated in 50 mM Tris-HCl (pH, 7.6) containing 0.03% DAB, 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide for 5 min. After BrdU labeling, a mouse antineuronal nucleic antibody (1: 1,000; Chemicon International, Temecula, CA, USA) was used on the same sections to differentiate neurons. The sections were mounted onto gelatin-coated slides, air-dried overnight at room temperature, and the coverslips were mounted using Permount (Thermo Fisher Scientific Inc.).
Immunohistochemistry for DCX
Immunohistochemistry for DCX was performed as previously described method (Park et al., 2019). The sections were incubated in PBS for 10 min, washed three times in PBS, and then incubated in 1% hydrogen peroxide for 20 min. The sections were incubated 2 hr with goat anti-DCX antibody (1:1,000; Oncogene Research Product). The sections were then incubated with the biotinylated goat secondary antibody (1:500; Vector Laboratories) for another 1 hr, washed, and incubated in ABC complex (Vector Elite ABC kit; 1:100; Vector Laboratories). Labeling was visualized using 0.03% DAB, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount (Thermo Fisher Scientific Inc.)
TUNEL staining
To visualize DNA fragmentation, we performed TUNEL staining using an In Situ Cell Death Detection Kit (Roche) according to the manufacturer’s protocol as previously described method (Song et al., 2018). The sections were postfixed in ethanol-acetic acid (2:1) and rinsed, then incubated with proteinase K (100 mg/mL) and rinsed again. They were then incubated in 3% hydrogen peroxide, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.03% DAB, counterstained with Nissl and mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature and the coverslips were mounted using Permount (Thermo Fisher Scientific Inc.).
Western blotting for BDNF, TrkB, Bax, and Bcl-2
Western blotting for the determination of Bax, Bcl-2, BDNF, TrkB was conducted as previously described method (Park et al., 2019; Song et al., 2018). The hippocampus tissues were homogenized on ice and lysed in a lysis buffer containing 50 mM Tris-HCl (pH, 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, and 100-mg/mL leupeptin. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein of 30 μg was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane, which was incubated with mouse β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse Bax (1:1,000; Santa Cruz Biotechnology), Bcl-2 (1:1,000; Santa Cruz Biotechnology), and rabbit BDNF (1:1,000; Santa Cruz Bio-technology), TrkB (1:1,000; Santa Cruz Biotechnology). Horseradish peroxidase-conjugated anti-mouse for β-actin, Bax, Bcl-2 and anti-rabbit for BDNF, TrkB were used as secondary antibodies.
Data analysis
For confirming the expression of Bax, Bcl-2, BDNF, and TrkB, the detected bands were calculated densitometrically using Molecular Analyst version 1.4.1 (Bio-Rad). The numbers of 5-HT-positive and TPH-positive cells in the dorsal raphe and DCX- and BrdU-positive cells in the hippocampal dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan). The data were analyzed with one-way analysis of variance and then Duncan post hoc test. All values are expressed as the mean±standard error of the mean (SEM), and P-value of <0.05 was considered significant.
RESULTS
Effect of swimming exercise on anxiety, depression, and short-term memory
Regarding anxiety in the elevated plus-maze test, the rats in the SI group showed anxiety compared to the GH group in open times and entries. Swimming exercise decreased anxiety level in the SI group (Fig. 1A, B). For depression in the forced swimming test, the rats in the SI group showed depression compared to the GH group in the immobility times. Swimming exercise alleviated depression level in the SI group (Fig. 1C). For short-term term memory in the step-through test, the rats in the SI group showed short-term memory impairment compared to the GH group in latency times. Swimming exercise improved short-term memory in the SI group (Fig. 1D).
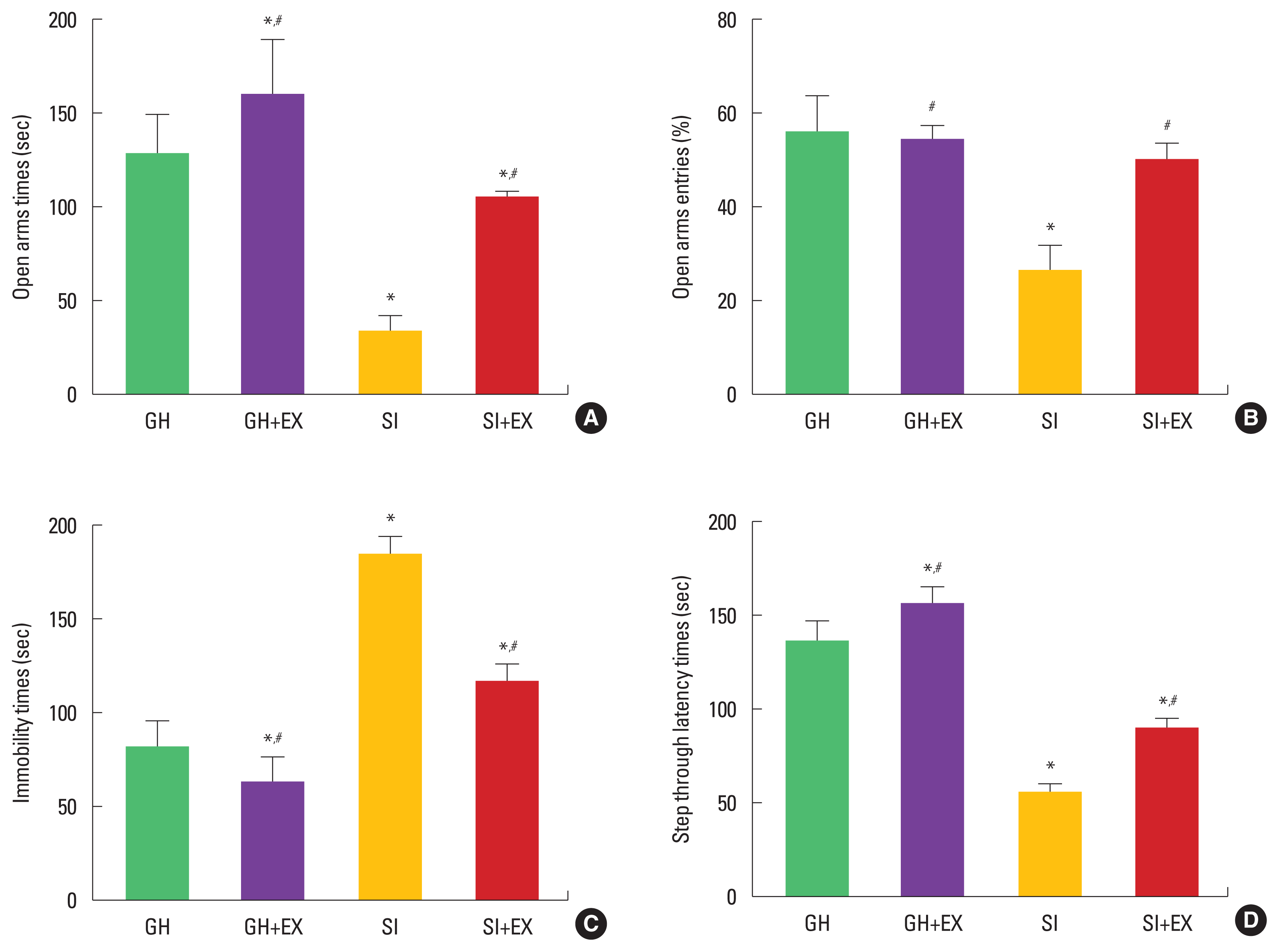
Effect of swimming exercise on behaviors. Open arms times in elevated plus-maze test (A), open arms entries in elevated plus-maze test (B), immobility times in forced swimming test (C), latency times in step-through avoidance task (D). GH, group housed; GH+EX, group housed and exercise; SI, socially isolated; SI+EX, socially isolated and exercise. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the GH group. #P<0.05 compared to the SI group.
Effect of swimming exercise on apoptosis in the hippocampus
When the expression of Bax and Bcl-2 in GH group was set at 1.00, the SI group showed an increase in Bax and a decrease in the Bcl-2 compared to the GH group. Swimming exercise decreased Bax expression and increased Bcl-2 expression in the SI group (Fig. 2A). The number of TUNEL-positive cells in the hippocampal dentate gyrus was increased in the SI group compared to the GH group. Swimming exercise decreased this number in the SI group (Fig. 2B).

Effect of swimming exercise on apoptosis in hippocampus. Bax and Bcl-2 (A), TUNEL-positive cells (B). The scale bar represents 50 μm. GH, group housed; GH+EX, group housed and exercise; SI, socially isolated; SI+EX, socially isolated and exercise. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the GH group. #P<0.05 compared to the SI group.
Effect of swimming exercise on BDNF and TrkB expression in the hippocampus
When the expression of BDNF and TrkB in GH group was set at 1.00, the SI group showed a decrease in BDNF and TrkB expression compared to the GH group. Swimming exercise increased BDNF and TrkB expression in the SI group (Fig. 3).
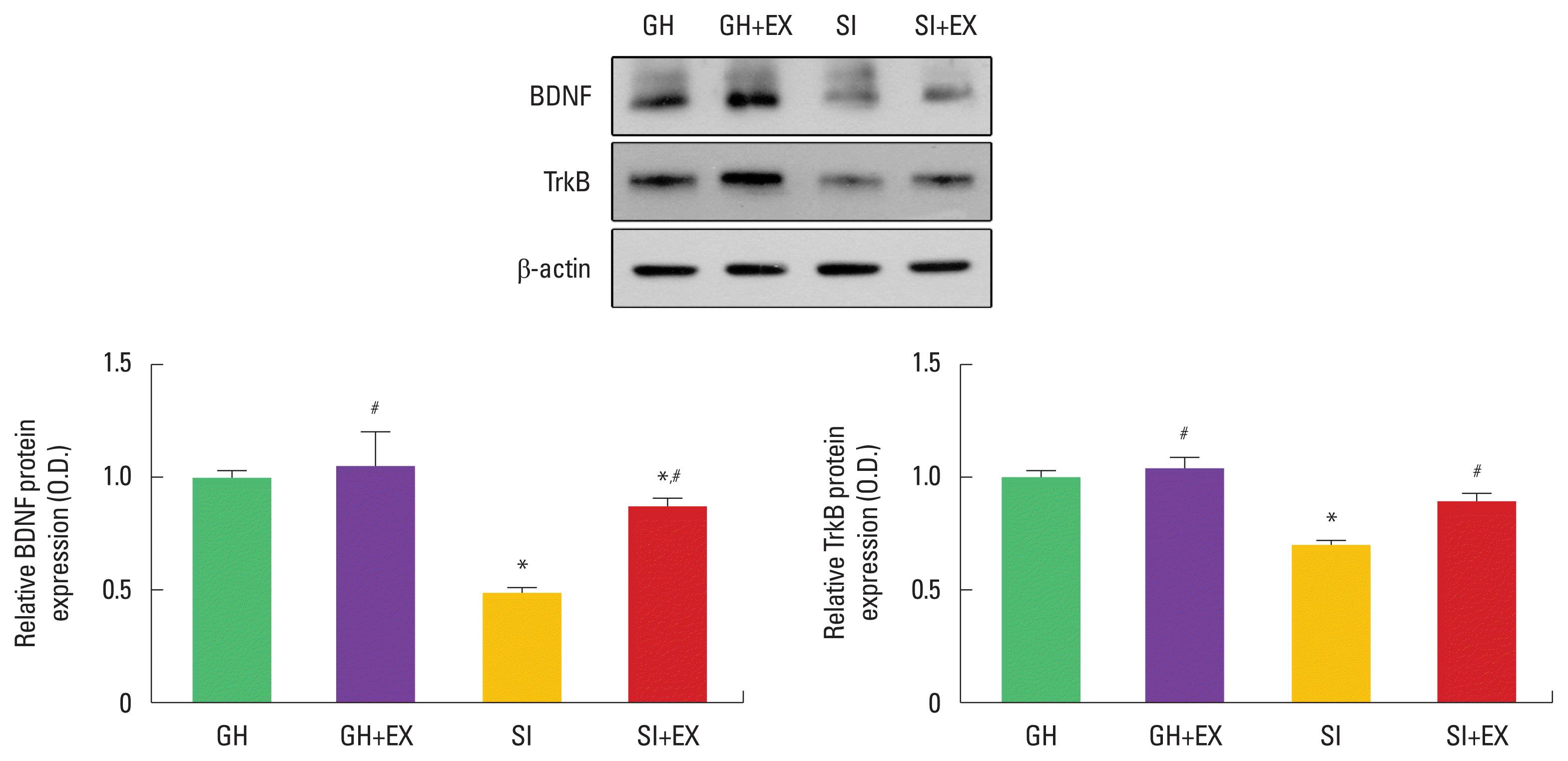
Effect of exercise on brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) in hippocampus. GH, group housed; GH+EX, group housed and exercise; SI, socially isolated; SI+EX, socially isolated and exercise. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the GH group. #P<0.05 compared to the SI group.
Effect of swimming exercise on cell proliferation and differentiation in hippocampal dentate gyrus
The number of BrdU-positive and DCX-positive cells in the hippocampal dentate gyrus was decreased in the SI group compared to the GH group. Swimming exercise increased these numbers in the SI group (Fig. 4).
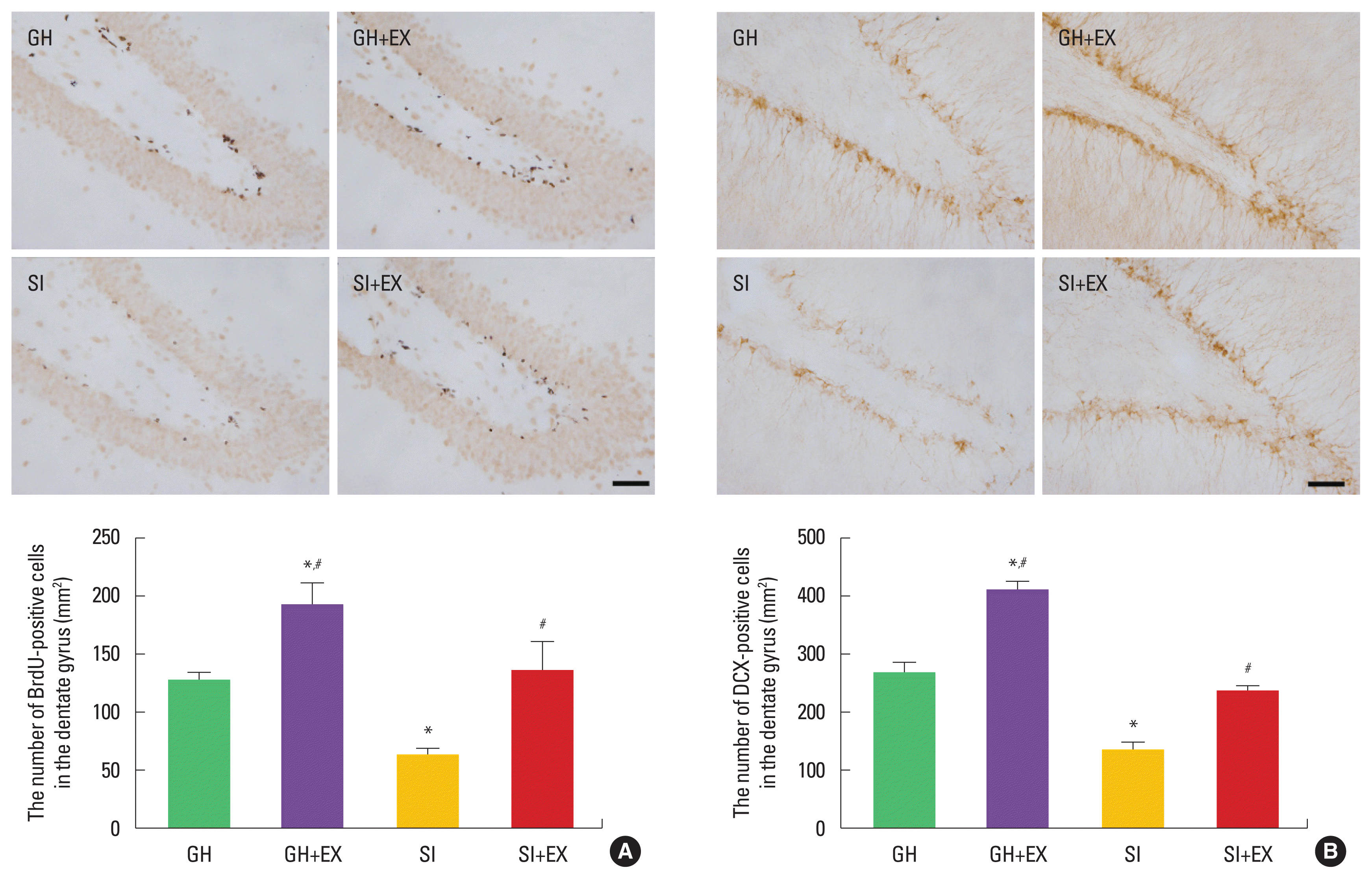
Effect of exercise on cell proliferation and differentiation in hippocampal dentate gyrus. 5-bromo-2′-deoxyuridine (BrdU)-positive cells (A), doublecortin (DCX)-positive cells (B). The scale bar represents 50 μm GH, group housed; GH+EX, group housed and exercise; SI, socially isolated; SI+EX, socially isolated and exercise. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the GH group. #P<0.05 compared to the SI group.
Effect of exercise on TPH and 5-HT in the dorsal raphe
The number of TPH-positive and 5-HT-positive cells in the dorsal raphe was decreased in the SI group compared to the GH group. Swimming exercise increased these numbers in the SI group (Fig. 5).
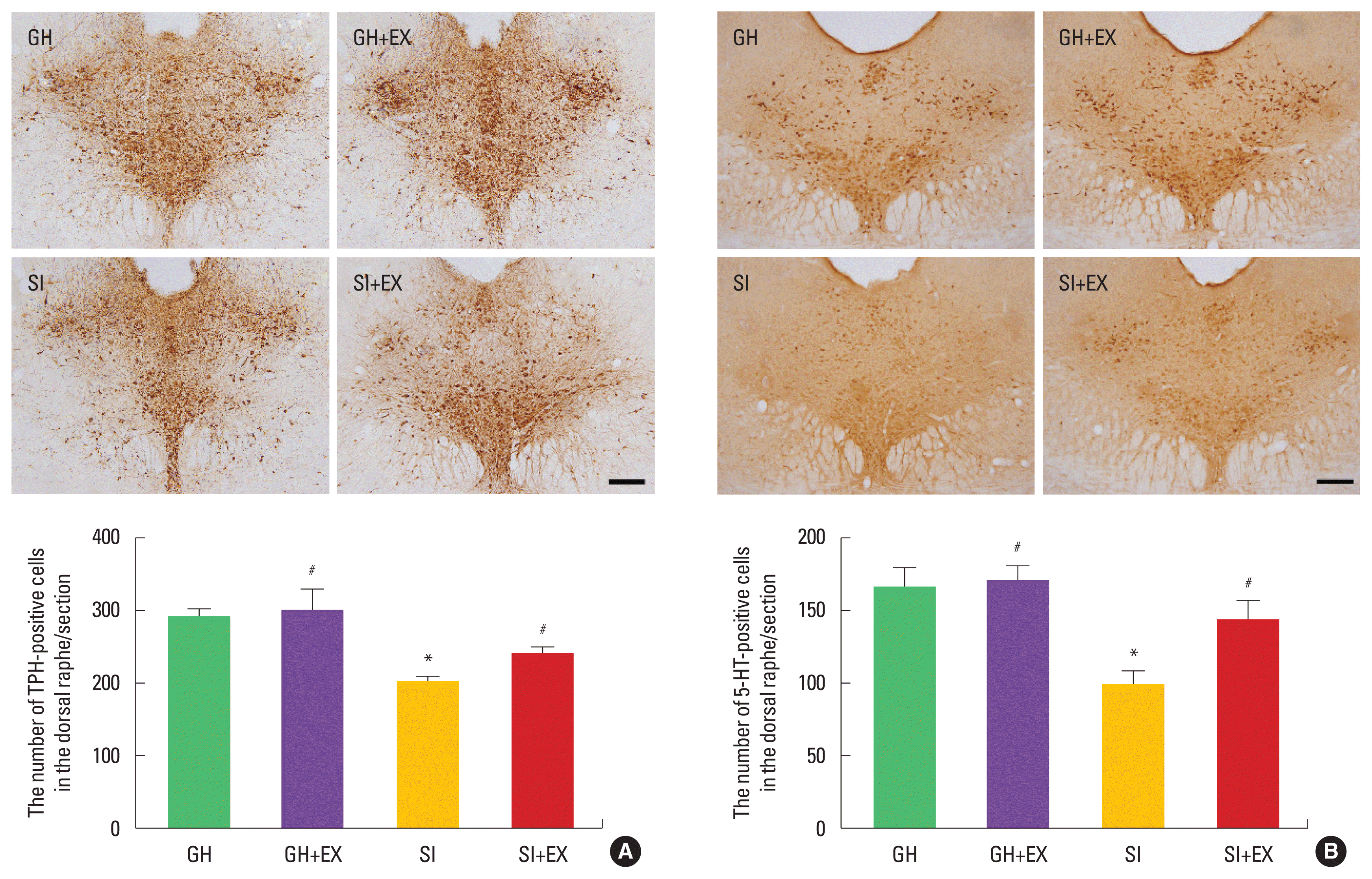
Effect of swimming exercise on serotonin expression in the dorsal raphe. Tryptophan hydroxylase (TPH)-positive cells (A), 5-hydroxytryptamine (5-HT)-positive cells (B). The scale bar represents 50 μm. GH+EX, group housed and exercise; SI, socially isolated; SI+EX, socially isolated and exercise. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the GH group. #P<0.05 compared to the SI group.
DISCUSSION
Social isolation during adolescence induced serious damage to the brain, leading to reduction in learning ability and spatial memory, causes behavioral change (Larsson et al., 2002). Social isolation decreased memory performance, declines memory capacity, increases negativity, and increased anxious and depressive behaviors (Cacioppo and Hawkley, 2009; Ma et al., 2011). Nitrergic system involved in the behavioral deficits, leading to the development of depression and anxiety (Amiri et al., 2015). In this study, the rats in the social isolation during adolescence stage showed anxiety in elevated plus-maze test, depression in forced swimming test, short- term memory impairment in step-through avoidance test.
Social isolation and chronic unpredictable mild stress exhibited depressive-like behavior and show a phenotype of mixed apoptosis/autophagy characteristic (Wang et al., 2019). In this study, social isolation during adolescence stage increased Bax expression, decreased Bcl-2 expression, and increased the number of TUNEL-positive cells. These results represent social isolation facilitated apoptosis.
Social isolation significantly reduced BDNF concentration in the hippocampus (Scaccianoce et al., 2006). In this study, social isolation during adolescence stage suppressed BDNF and TrkB expression in the hippocampus.
Social isolation promoted expression of depression-related parameters, which inhibit generation of neurons (Huang et al., 2011). Social isolation stress factors changed neurogenesis in the hippocampus, which could impair spatial memory in adulthood (McCormick et al., 2012). The effect of stress on hippocampal neurogenesis and associated behaviors might be particularly strong during adolescence (Kozareva et al., 2018). In this study, social isolation during adolescence stage suppressed the number of BrdU-positive and DCX-positive cells in the hippocampal dente gyrus. These results represent social isolation inhibited cell proliferation and differentiation.
Social isolation in the adolescent period increased serotonin release in the nucleus accumbens (Lukkes et al., 2009). The activation of 5-HT neurons was vulnerable to stress and played an important role in behaviors related to depression and anxiety (Teissier et al., 2015). Social isolation during the adolescent period resulted in decreased excitability of 5-HT neurons in the dorsal raphe (Sargin et al., 2016). In this study, social isolation during adolescence stage inhibited TPH and 5-HT expression in the dorsal raphe.
Exercise after social isolation increased the level of BDNF in the hippocampus and improve depression by increasing serotonergic cells in the raphe nuclei (Hong et al., 2015). Exercise improved mood disorders, including depression and anxiety, and reduces depressive symptoms by increased serotonin expression (Ji et al., 2017; Park et al., 2017). Exercise enhanced hippocampal neurogenesis during adolescence (Kozareva et al., 2018).
In this study, swimming exercise ameliorated anxiety, depression, short-term impairment. Swimming exercise suppressed apoptosis, enhanced neurogenesis, and increased serotonin expression. In our study, swimming exercise ameliorates mood disorder and memory impairment by enhancing neurogenesis and serotonin expression and inhibiting apoptosis in social isolation during adolescence.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A5A2A01027017).
