Treadmill exercise ameliorates memory impairment through ERK-Akt-CREB-BDNF signaling pathway in cerebral ischemia gerbils
Article information
Abstract
Neuronal cell death in the hippocampus by cerebral ischemia causes disability of memory function. Cerebral ischemia also alters the expressions of brain-derived neurotrophic factor (BDNF), cyclic adenosine monophosphate-responsive element binding protein (CREB), extracellular signal-regulated protein kinase (ERK), and phosphatidylinositol 3-kinase/protein kinase B (Akt). In the present study, we investigated the effect of treadmill exercise on cerebral ischemia in relation with ERK-Akt-CREB-BDNF signaling pathway in the hippocampus using gerbils. Induction of cerebral ischemia deteriorated short-term memory with suppression of phosphorylation of ERK-Akt-CREB-BDNF pathway in the hippocampus of gerbils. Enhancement of apoptosis in the hippocampus was accompanied in the ischemia gerbils. Treadmill exercise improved short-term memory through enhancing phosphorylation of ERK-Akt-CREB-BDNF pathway with suppressing apoptosis in the hip-pocampus of the ischemia gerbils. The present results suggest that improvement of memory function after cerebral ischemia by treadmill exercise may be involved in the ERK-Akt-CREB-BDNF signaling pathway, resulting in inhibition of apoptosis in the hippocampus.
INTRODUCTION
Neuronal cell death in the hippocampus by cerebral ischemia causes disability of memory function. Cerebral ischemia also alters the expressions of brain-derived neurotrophic factor (BDNF), cyclic adenosine monophosphate-responsive element binding protein (CREB), extracellular signal-regulated protein kinase (ERK), and phosphatidylinositol 3-kinase (PI3-kinase)/protein kinase B (Akt) (Irving and Bamford, 2002; Ko et al., 2018; Wang et al., 2009; Zhu et al., 2004).
BDNF is a member of the neurotrophic family involved in neuronal survival and differentiation, and it plays an important role in learning process due to its involvement in long-term potentiation in the hippocampus (Yamada et al., 2002). BDNF is a CREB target gene and plays a crucial role in the synaptic plasticity of the brain (Poo, 2001). BDNF is involved in neuronal survival and activates ERK, CREB, and Akt after binding to its receptor tyrosine kinase B (TrkB) (Numakawa et al., 2010). Activation of TrkB for BDNF triggers several parallel signaling pathways, including the Ras/ERK, PI3-kinase (Santos et al., 2010). BDNF is known to exert protective effect against ischemic insult (Ko et al., 2018).
CREB-mediated transcription system is necessary for the induction of ischemic tolerance after global ischemia (Hara et al., 2003). CREB is associated with neuronal survival, memory consolidation, and synaptic plasticity (Impey et al., 2004). CREB activation is a critical event in neuroprotection against ischemic injury (Meller et al., 2005).
ERK is one of the mitogen-activated protein kinase families and modulates cell growth, differentiation, and survival. Reduced ERK signaling contributes to neuronal cell death in hippocampal dentate after transient cerebral ischemia (Yan et al., 2007). BDNF/TrkB activates ERK-regulated CREB phosphorylation (Whitfield et al., 2011).
Apoptosis after cerebral ischemia is one of the major pathways that lead to the process of cell death (Mattson et al., 2001). Two important groups of proteins involved in ischemic apoptotic cell death are the members of the Bcl-2 family and a class of cysteine proteases known as caspases (Choi et al., 2017; Park et al., 2016). Bcl-2 family is classified into two functionally distinct groups: Bcl-2, an anti-apoptotic protein, and Bax, a pro-apoptotic protein. Caspase-3 is one of the most widely studied members of the caspase family, and it is one of the key executors of apoptosis.
Memory improving and antiapoptotic effects of exercise are well documented (Ko et al., 2018; Lee et al., 2019). In the present study, we investigated the effect of treadmill exercise on cerebral ischemia in relation with ERK-Akt-CREB-BDNF signaling pathway in the hippocampus using gerbils.
MATERIALS AND METHODS
Experimental animals
Male Mongolian gerbils weighing 60±10 g were used in this experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The gerbils were randomly assigned into four groups (n=10 in each group): the sham-operation group, the sham-operation and exercise group, the ischemia-induction group, and the ischemia-induction and exercise group.
Induction of transient global ischemia
Transient global ischemia was induced according to the previously described surgical procedure (Choi et al., 2017; Lee et al., 2019). The gerbils were anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France). Following bilateral neck incision, both common carotid arteries were exposed and occluded with aneurysm clips for 5 min. The clips were then removed to restore cerebral blood flow. Body and rectal temperature were maintained at 36°C±0.5°C during surgery using Homeothermic Blanket Control Unit (Harvard Apparatus, Massachusetts, MA, USA).
Treadmill exercise protocol
The gerbils in the treadmill exercise groups were subjected to run on a treadmill for 30 min once a day for 14 days, starting one day after induction of cerebral ischemia. The exercise load consisted of running at a speed of 2 m/min for the first 5 min, 3 m/min for the next 5 min, and 4 m/min for the last 20 min with the 0° inclination.
Step-down avoidance task
In order to evaluate short-term memory in the gerbils, the latency in the step-down avoidance task was determined as the previously described method (Kim et al., 2019; Ko et al., 2019). On the 12th day starting treadmill exercise, the gerbils were trained in a step-down avoidance test. The gerbils were positioned on a 7×25-cm platform with a height of 2.5 cm, and then allowed to rest on the platform for 2 min. The platform faced a 42×25-cm grid of parallel 0.1-cm-caliber stainless steel bars, which were spaced 1 cm apart. In the training sessions, the animals received a 0.5-mA scramble foot shock for 3 sec immediately upon stepping down. Retention time was assessed 1 day after training session. The interval for gerbils stepping down and placing all four paws on the grid was defined as the latency in the step-down avoidance task. The latency over 300 sec was counted as 300 sec.
Tissue preparation
For brain tissue preparation, the animals were fully anesthetized with Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline, and fixed with freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (pH, 7.4). Brains were then removed, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40-μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
Caspase-3 immunohistochemistry
Caspase-3 immunohistochemistry was performed according to a previously described method (Park et al., 2019; Song et al., 2018). The sections were incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then they were incubated for another 1 hr with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified using a Vector Elite ABC kit (1:200; Vector Laboratories, Burlingame, CA, USA). The antibody-biotin-avidin-peroxidase complex was visualized using 0.02% diaminobenzidine. The sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount (Fisher Scientific, Pittsburgh, PA, USA).
Western blot analysis
Western blot analysis was performed, as the previously described method (Kim et al., 2019; Park et al., 2019; Song et al., 2018). Brain tissues were lysed in an ice-cold whole cell lysate buffer. Protein of 30 μg was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane (Whatman, Clifton, NJ, USA). The nitrocellulose membrane was blocked by incubation for 90 min at room temperature in tris-buffered saline containing 0.1% tween 20 and 5% nonfat dried milk (Becton Dickinson, Sparks, MD, USA). Rabbit glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody (1:10,000; Affinity Bioreagent, Rockford, IL, USA), rabbit BDNF, TrkB, and phosphorylated- PI3-kinase (p-PI3-kinase) antibody (1:1,000; Santa Cruz Biotechnology), rabbit Akt and phosphorylated-Akt (p-Akt) antibody (1:1,000; Cell Signaling Technology, Beverly, MA, USA), mouse Bax, Bcl-2, PI3-kinase, ERK and phosphorylated-ERK (p-ERK) antibody (1:1,000; Santa Cruz Biotechnology), rabbit CREB (1: 1,000; Millipore, Billerica, MA, USA), and goat phosphorylated- CREB antibody (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti- rabbit antibody for GAPDH, BDNF, TrkB, CREB, p-PI3-kinase, Akt, and p-Akt (1:3,000; Vector Laboratories), horseradish peroxidase-conjugated anti-mouse antibody (1:3,000; Vector Laboratories) for Bax, Bcl-2, PI3-kinase, ERK and p-ERK, and horseradish peroxidase-conjugated anti-goat antibody (1:5,000; Amersham Pharmacia Biotechnology GmbH, Freiburg, Germany) for p-CREB were used as the secondary antibodies. Experiments were performed under normal laboratory conditions and at room temperature, except for the transfer to membranes. The transfer was performed at 4°C with a cold pack and prechilled buffer. Band detection was performed using enhanced chemiluminescence detection kits (Santa Cruz Biotechnology).
Data analysis
The area of the hippocampal CA1 region was measured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA). The numbers of caspase-3-positive cells in the hippocampus were counted hemilaterally and the data are expressed as the number of cells per mm2 of the hippocampus. To compare the relative expressions of BDNF, TrkB, ERK, p-ERK, Akt, p-Akt, CREB, p-CREB, Bax, and Bcl-2, the detected bands were calculated densitometrically using Image-Pro Plus software (Media Cybernetics). Statistical analysis was performed using one-way analysis of variance followed by Duncan post hoc test. The results are presented as the mean±standard error of the mean. Significance was set as P<0.05.
RESULTS
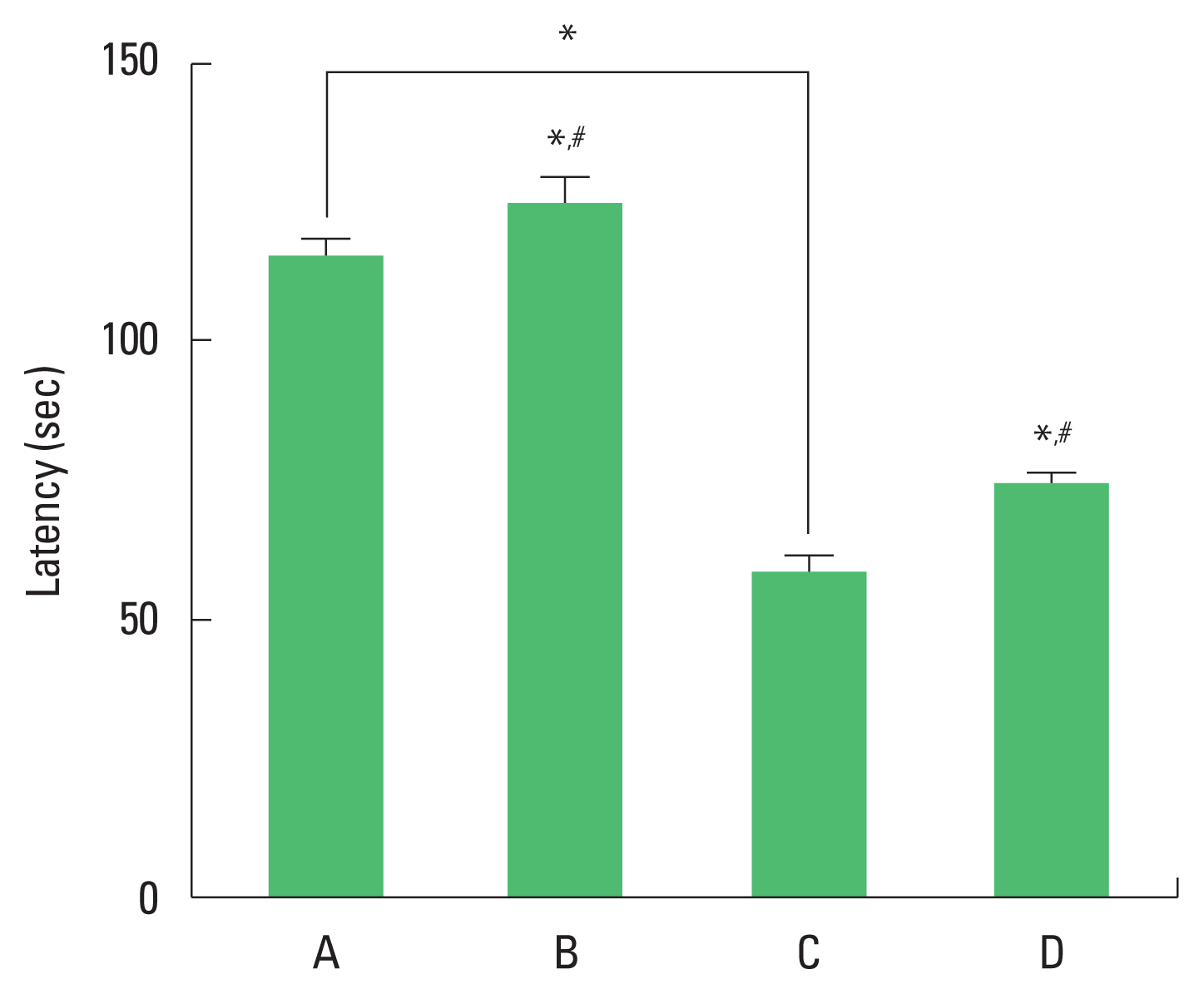
Short-term memory
The results of the step-through avoidance task are presented in Fig. 1. Short-term memory was impaired by ischemia induction. Treadmill exercise ameliorated ischemia-induced memory impairment.
Expression of BDNF and TrkB in the hippocampus
The results of the relative expression of BDNF and TrkB in the hippocampus are presented in Fig. 2. BDNF and TrkB expression were decreased by ischemia induction. Treadmill exercise increased BDNF and TrkB expression in the ischemia gerbils.
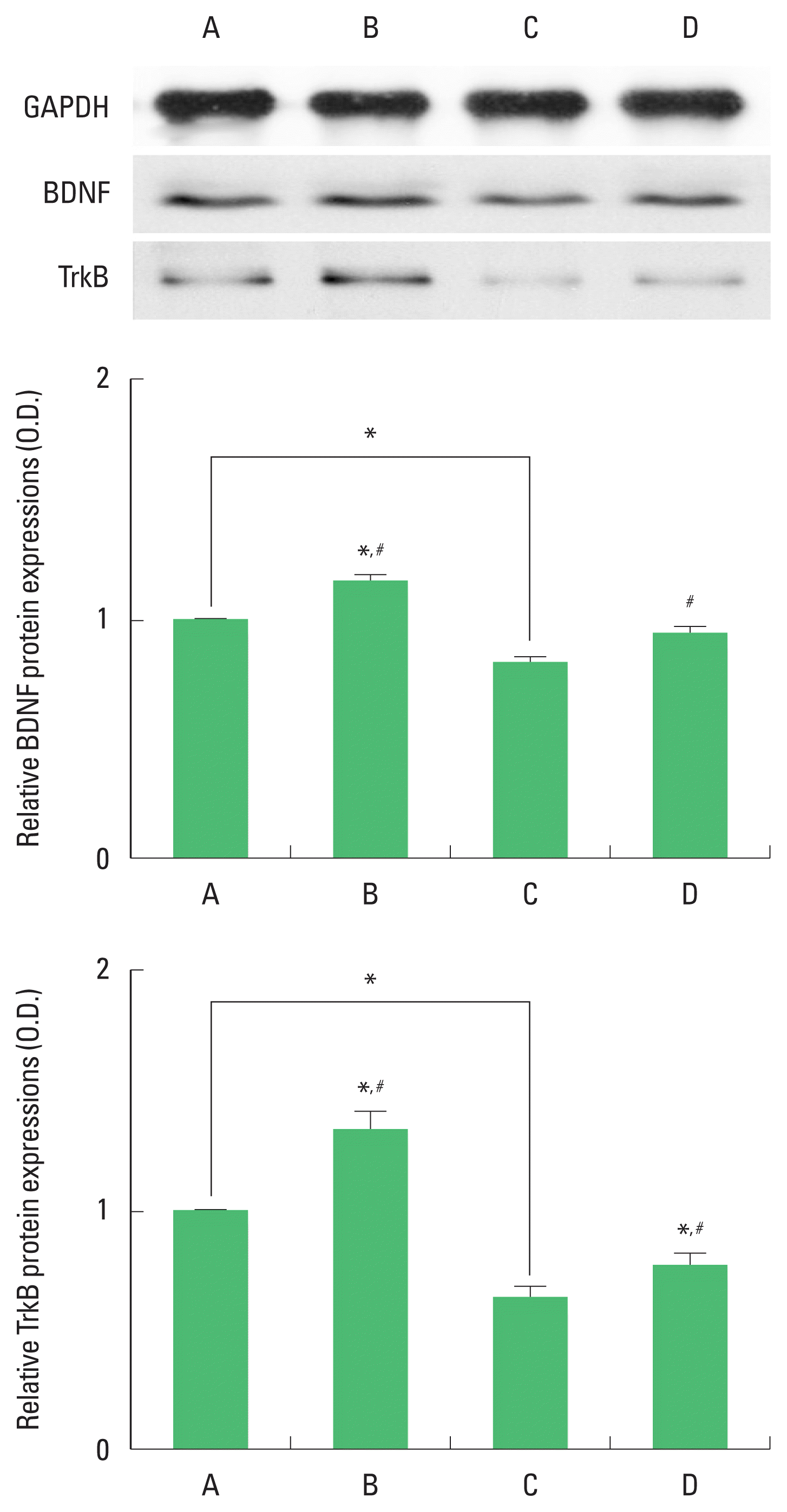
Brain-derived neurotrophic factor (BDNF) and tyrosine kinase B (TrkB) expression in the hippocampus. Upper panel: representative expression of BDNF and TrkBn. Middle and lower panel: relative expressions of BDNF and TrKB. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of CREB and p-CREB in the hippocampus
The results of the relative expression of CREB and p-CREB in the hippocampus are presented in Fig. 3. p-CREB expression was decreased by ischemia induction, resulting in decrease of p-CREB/CREP ratio. Treadmill exercise increased p-CREB expression in the ischemia gerbils, resulting in increase of p-CREB/CREP ratio.
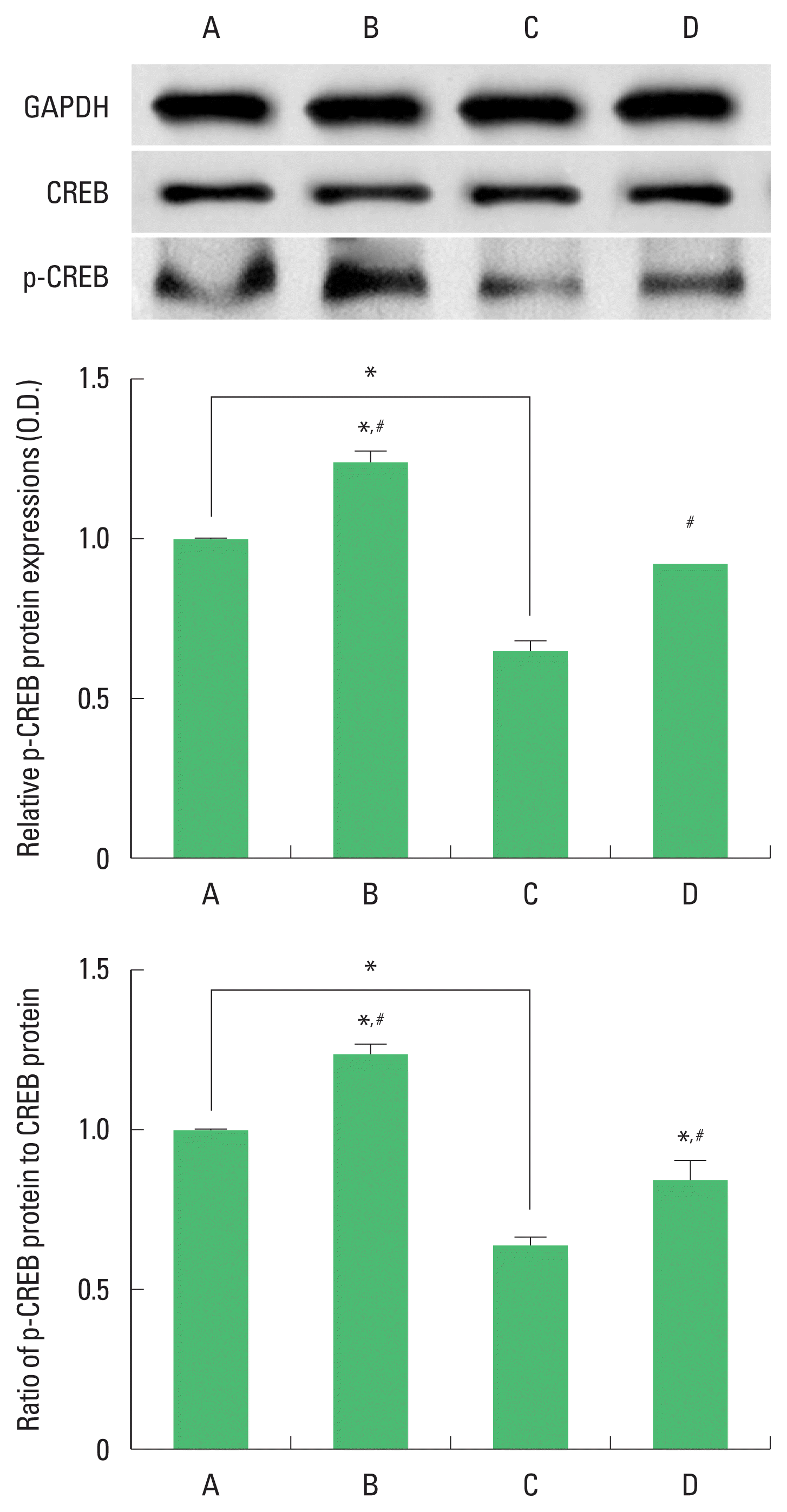
Cyclic adenosine monophosphate-responsive element binding protein (CREB) and phosphorylated CREP (p-CREB) expression in the hippocampus. Upper panel: representative expression of CREP and p-CREB. Middle and lower panel: relative expressions of CREP and p-CREB. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of ERK and p-ERK in the hippocampus
The results of the relative expression of ERK and p-ERK in the hippocampus are presented in Fig. 4. p-ERK expression was decreased by ischemia induction, resulting in decrease of p-ERK/ERK ratio. Treadmill exercise increased p-ERK expression in the ischemia gerbils, resulting in increase of p-ERK/ERK ratio.

Extracellular signal-regulated protein kinase (ERK) and phosphorylated-ERK (p-ERK) expression in the hippocampus. Upper panel: representative expression of ERK and p-ERK. Middle and lower panel: relative expressions of ERK and p-ERK. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of Akt and p-Akt in the hippocampus
The results of the relative expression of Akt and p-Akt in the hippocampus are presented in Fig. 5. p-Akt expression was decreased by ischemia induction, resulting in decrease of p-Akt/Akt ratio. Treadmill exercise increased p-Akt expression in the ischemia gerbils, resulting in increase of p-Akt/Akt ratio.
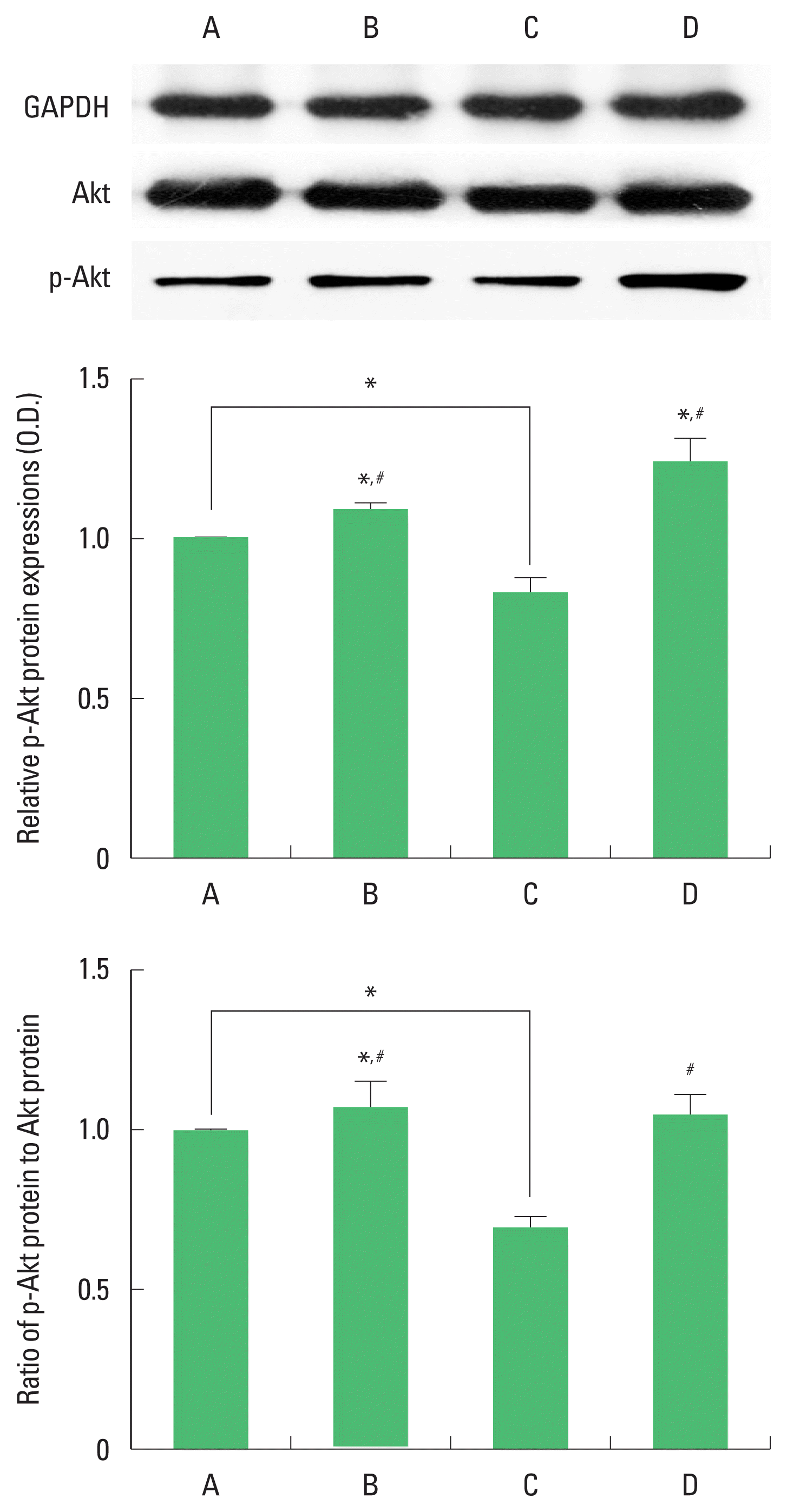
Protein kinase B (Akt) and phosphorylated-Akt (p-Akt) expression in the hippocampus. Upper panel: representative expression of Akt and p-Akt. Middle and lower panel: relative expressions of Akt and p-Akt. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of PI3-kinase and p-PI3-kinase in the hippocampus
The results of the relative expression of PI3-kinase and p-PI3-kinase in the hippocampus are presented in Fig. 6. p-PI3-kinase expression was decreased by ischemia induction, resulting in decrease of p-PI3-kinase/PI3-kinase ratio. Treadmill exercise increased p-PI3- kinase expression in the ischemia gerbils, resulting in increase of p-PI3-kinase/PI3-kinase ratio.

Phosphatidylinositol 3-kinase (PI3-kinase) and phosphorylated PI3-kinase (p-PI3-kinase) expression in the hippocampus. Upper panel: representative expression of PI3-kinase and p-PI3-kinase. Middle and lower panel: relative expressions of PI3-kinase and p-PI3-kinase. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of caspase-3 in the hippocampal CA1 region
The results of the expression of caspase-3 in the hippocampal CA1 region are presented in Fig. 7. Caspase-3 expression was decreased by ischemia induction. Treadmill exercise increased caspase-3 expression in the ischemia gerbils.

Caspase-3 expression in the hippocampal CA1 region. Upper panel: photomicrographs of caspase-3-positive cells. The scale bar represents 50 μm. Lower panel: number of caspase-3-positive cells in each group. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
Expression of Bax and Bcl-2 in the hippocampus
The results of the relative expression of Bax and Bcl-2 in the hippocampus are presented in Fig. 8. Bax expression was increased and Bcl-2 expression was decreased by ischemia induction, resulting in increase of Bax/Bcl-2 ratio. Treadmill exercise decreased Bax expression and increased Bcl-2 expression in the ischemia gerbils, resulting in decrease of Bax/Bcl-2 ratio.
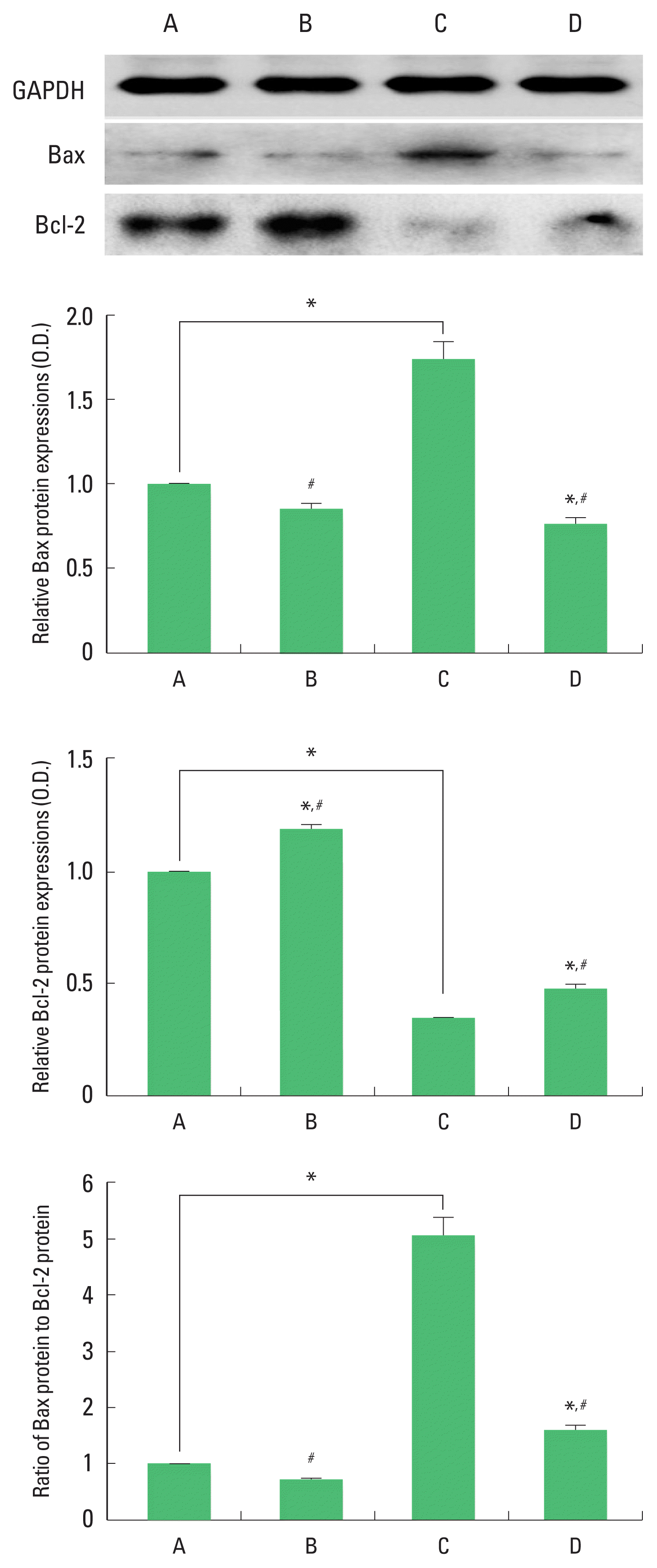
Bax and Bcl-2 expression in the hippocampus. Upper panel: representative expression of Bax and Bcl-2. Middle and lower panel: relative expressions of Bax, Bcl-2, and Bax to Bcl-2 ratio. A, sham-operation group; B, sham-operation and exercise group; C, ischemia-induction group; D, ischemia-induction and exercise group. *P<0.05 compared to the sham-operation group. #P<0.05 compared to the ischemia-induction group.
DISCUSSION
Hypoxic-ischemic brain damage deteriorated short-term memory in rats (Park et al., 2016) and ischemia impaired short-term memory in gerbils (Choi et al., 2017). Treadmill exercise improved memory function through suppressing apoptosis in the hippocampus following cerebral ischemia (Choi et al., 2017; Park et al., 2016). In this study, the latency in the step-down avoidance task was decreased by induction of ischemia, indicating that ischemia deteriorated short-term memory in gerbils. In contrast, treadmill exercise enhanced the latency in the step-down avoidance task.
BDNF exerts its neuroprotective effect against ischemic injury via activation of the ERK signaling pathway (Han and Holtzman, 2000). Furthermore, caspase-3 is inhibited by increasing BDNF expression in cerebral ischemia (Cao et al., 2011; Choi et al., 2017). The protective effect of exercise on brain injury is due to the activation of BDNF signaling pathway by exercise (Ko et al., 2018). In the present results, BDNF and TrkB expression in the hippocampus was suppressed by the induction of ischemia. In contrast, treadmill exercise enhanced BDNF and TrkB expression in the ischemia gerbils.
CREB-dependent expression of BDNF was increased by exercise (Tokuyama et al., 2000). CREB is an important transcription factor in the BDNF-TrkB signaling and its phosphorylation form of CREB (p-CREB) mediates neurogenesis in the adult hippocampal dentate gyrus after focal ischemia (Zhu et al., 2004). In the present study, induction of cerebral ischemia decreased phosphorylation of CREB in the hippocampus. In contrast, treadmill exercise increased phosphorylation of CREB in the hippocampus of ischemia gerbils.
PI3-kinase phosphorylates the 3′-hydroxyl group of phosphatidylinositols. This reaction leads to the activation of many intracellular signaling pathways that regulate functions as diverse as cell metabolism, survival, polarity, and vesicle trafficking (Knight et al., 2006). Akt acts downstream of PI3-kinase to regulate many biologic process such as proliferation, apoptosis, and growth. Akt plays an important role in cell survival signaling of the brain and it exerts a neuroprotective response to ischemia (Fukunaga and Kawano, 2003). Induction of global ischemia decreased Akt phosphorylation in the hippocampal CA1 (Koh et al., 2006). Neuroprotective role of the phosphorylation form of PI3-kinase/Akt pathway following cerebral ischemia is well documented (Carloni et al., 2010; Wang et al., 2009). Activated Akt inhibits apoptosis by phosphorylating its substrates, such as Bcl-2-associated death proteins (Hanada et al., 2004). Exercise-induced cognitive improvement is mediated by phosphorylation of PI3-kinase/Akt (Chae and Kim, 2009). In the present study, induction of cerebral ischemia suppressed phosphorylation of Akt and PI3-kinase in the hippocampus. In contrast, treadmill exercise enhanced phosphorylation of Akt and PI3-kinase in the hippocampus of ischemia gerbils.
ERK is involved in the regulating brain cell death and survival after ischemia (Irving and Bamford, 2002). Elevated ERK phosphorylation in the brain initiates a downstream signaling cascade, including CREB and BDNF, which initiates the positive feedback (Lonze and Ginty, 2002). The ERK-CREP-BDNF signaling cascade plays a crucial role in the learning and memory performance (Ying et al., 2002). In the present study, induction of cerebral ischemia decreased phosphorylation of ERK in the hippocampus. In contrast, treadmill exercise increased phosphorylation of ERK in the hippocampus of ischemia gerbils.
Apoptosis after cerebral ischemia is the final event that leads to the process of cell death (Mattson et al., 2001). Ischemia triggers apoptotic neurodegeneration through caspase-3 activation (Choi et al., 2017; Park et al., 2016). In the ischemic attack, increased expression of Bcl-2 inhibited apoptosis, while over-expression of Bax promoted apoptosis (Choi et al., 2017; Park et al., 2016). Increase of Bax to Bcl-2 ratio represents apoptotic cell death (Song et al., 2018). In the present study, caspase-3 expression in the hippocampal CA1 region was enhanced by the induction of cerebral ischemia insult. In contrast, treadmill exercise suppressed caspase-3 expression in the hippocampal CA1 region of ischemia gerbils. Bax expression was increased and Bcl-2 expression was decreased in the hippocampus following cerebral ischemia. As a result, induction of cerebral ischemia increased the ratio of Bax to Bcl-2. In contrast, treadmill exercise suppressed Bax expression and enhanced Bcl-2 expression in the hippocampus of ischemia gerbils, resulting in decrease of Bax to Bcl-2 ratio.
In this study, treadmill exercise improved short-term memory through increasing ERK and CREB phosphorylation with activation of Akt signaling with its downstream of BDNF-TrkB signaling in the cerebral ischemia gerbils. These results suggest that improvement of memory function after cerebral ischemia by treadmill exercise may be involved in the ERK-Akt-CREB-BDNF signaling pathway, resulting in inhibition of apoptosis in the hippocampus.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2018S1 A5A2A01031391).