Effects of preconditioning exercise on nitric oxide and antioxidants in hippocampus of epileptic seizure
Article information
Abstract
The mechanism of epileptic seizure has not been identified clearly. Exercise can play a role of antioxidants against oxidative stress. In the present study, the neuroprotective effects of preconditioning exercise on epileptic seizure were investigated with focusing on antioxidants activity in the hippocampus. Rats were allocated to the following groups: saline control group, kainic acid control group, and previous exercise and kainic acid group. Rats in the previous exercise and kainic acid group were subjected to treadmill exercise 5 days a week for 4 weeks. After 48 hr of exercise period, rats in the kainic acid control group and previous exercise and kainic acid group were injected with kainic acid. The number of neuronal nitric oxide synthase-positive cells and the level of nitrite in hippocampus were increased and the expressions of superoxide dismutase-1, superoxide dismutase-2, and catalase in hippocampus were reduced in kainic acid control group compared with saline control group. By contrast, in the previous exercise and kainic acid group, the number of neuronal nitric oxide synthase-positive cells and the level of nitrite were decreased and the expressions of superoxide dismutase-1, superoxide dismutase-2, and catalase were increased compared with the kainic acid control group. Preconditioning exercise may have neuroprotective effects against oxidative stress via increased antioxidant activity in the hippocampus of epileptic seizure.
INTRODUCTION
The epileptic seizure that is neuronal disorder involves involuntary convulsion and the underlying mechanism has not been identified clearly. However, it has reported that neuronal death induced by dysfunction of neurotransmitter is one of crucial cause in epileptic seizure (Sommer et al., 2001). Especially, hippocampus is the main target region of neuronal cell death induced by repeated seizure, dysfunction of hippocampus is important etiologic pathophysiology of epileptic seizure (Sharma et al., 2007).
Excitotoxicity is induced by dysfunction of glutamate and excitotoxicity is the main mechanism of neuronal disorder by seizure in epilepsy (Beal, 1992). In excitotoxicity, abnormal hyperactivity of glutamate induces the hyper-expression of nitric oxide (NO) by neuronal nitric oxide synthase (nNOS), which influence neuronal damages of oxidative stress (Chen et al., 2014).
In many studies of epileptic seizure, kainic acid seizure model is useful for epileptic activity in the hippocampus (Kim et al., 2011; Wang et al., 2018). Kainic acid model reported oxidative stress and neuronal cell death in hippocampus with epileptic behaviors (Shin et al., 2008). Epileptic seizure is the response of exitotoxicitiy by oxidative stress (Shin et al., 2011).
Exercise has been demonstrated to have positive effects on brain function. Regular exercise improves neurogenesis, inhibits apoptosis, and increases neurotrophins (Fabel and Kempermann, 2008; Gomez-Pinilla and Hillman, 2013). In addition, exercise plays crucial role for controlling glutamate and activation of internal antioxidant enzyme system (Holmes et al., 2015; Ravi Kiran et al., 2004). In the study of Vanzella et al. (2017) using the aged animals, treadmill exercise improved enzyme ratio of superoxide dismutase (SOD) and catalase (CAT). These results showed that regular exercise can play a role of antioxidants against oxidative stress. Evidences indicate that effects of chronic exercise lasted for a while and protected with neuronal dysfunctions by subsequent neurodegenerative diseases (Aboutaleb et al., 2015; Zhao et al., 2015). These reports represented grounds that previous exercise conditioning can protect against hippocampal damage and disorder in epileptic seizure. However, it is nonexistent for the study that effects of preconditioning exercise about excitotoxicity by oxidative stress in epileptic seizure. In the present study, the neuroprotective effects of preconditioning exercise on epileptic seizure were investigated with focusing on antioxidant activity in the hippocampus.
MATERIALS AND METHODS
Animals
Male, 3-week-old Sprague-Dawley rats (n=30, Samtako Bio. Korea Co. Ltd., Seoul, Korea) were adapted to the laboratory environment (temperature, 22°C±1°C; relative humidity, 55%±3%; 12-hr light/12-hr dark photoperiod) for 2 weeks. All rats were housed in pairs, given free access to water and fed a standard chow diet. Studies were performed in accordance with Korea National Sport University standards for the Care and Use of Laboratory Animals (publication no. KNSU-IACUC-2017-05). Rats were allocated to the following groups: saline control group (SC; n=10), kainic acid control group (KA; n=10), and previous exercise and kainic acid group (KE; n=10).
Exercise protocol
After 2 weeks adaptation for environment, rats in the KE group were subjected to treadmill exercise 5 days a week for 4 weeks. The treadmill exercise was adapted low-intensity exercise that was increased gradually 5 m/min for first 5 min, 8 m/min next 5 min, and 11 m/min last 20 min.
Epileptic seizure
After 48 hr of exercise period, rats in the KA and KE groups were injected kainic acid (10 mg/kg/mL, intraperitoneally). After injection of kainic acid, the animals were put in cages and observed for 8 hr to evaluate involuntary seizure and response by contact stimulus.
Tissue preparation
After 24 hr of injection, the animals were sacrificed. For the immunohistochemistry, 5 rats of each group were anesthetized by an intraperitoneal (intraperitoneally) injection with xylazine (8 mg/kg) and ketamine (40 mg/kg). Rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution of 4% paraformaldehyde in 100 mM phosphate buffer (PB; pH, 7.4). The brains were dissected and post-fixed in the same fixative for 2 days, and then transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40-μm thickness were made using a freezing microtome (Leica, Nussloch, Germany). For the analysis of protein levels, brains were quickly extracted, and the hippocampus was dissected and stored at −70°C until analysis.
Immunohistochemistry
To detect nNOS-positive cells in the dentate gyrus, brain sections were transferred in 6-well plates loaded with 0.1 M PBS. Rinse sections twice, 10 min each rinse, with 0.1 M PBS on a shaker. After rinsing, sections were incubated with fresh 0.3% H2O2 in 0.1 M PBS for 30 min and then blocking solution (BSA 0.1 g; goat serum 1 mL; 0.1 M PBS 9 mL) for 60 min at room temperature. The sections were incubated 3 days with primary antibody (1:600, mouse anti-nNOS) diluted in blocking solution at 4°C for reducing background staining. The sections were then washed 3 times with PBS and incubated for 1 hr with a biotinylated anti-mouse secondary antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.03% DAB and 0.03% H2O2 for 5 min.
Subsequently, the slides were air-dried overnight at room temperature and coverslides were mounted using Permount. The number of nNOS-positive cells in the dentate gyrus of hippocampus was counted hemilaterally in every eighth section throughout the dentate gyrus at 400×magnification. The area of the dentate gyrus was traced using the Image Pro Plus image analyzer (Media Cybernetics Inc., Silver Springs, MD, USA) at 40×magnification. For the number of cells, volume of the dentate gyrus was calculated by means of the Cavalieri method as described (Llorens-Martín et al., 2006). The data of the number of cells are obtained by multiplying the mean cell density by the mean dentate gyrus volume for each individual separately and then obtaining the group mean. The mean cell density of each individual is calculated by using a standard optical dissector protocol as published previously (Trejo et al., 2001).
Western blotting
To prepare protein for western blotting, each hippocampus was crushed in a solution containing 150 mM NaCl, 5 mM EDTA, 50 mM Tris-HCl (pH 8.0), 1% NP-40, 1 mM aprotinin, 0.1 mM leupeptin and 1 mM pepstatin, and centrifuged at 15,294×g for 15 min at 4°C. Proteins were quantified by a Bradford assay and 30 μg was loaded onto a 10% gel, subjected to SDS-PAGE and transferred to a polyvinylidene difluoride membrane (EMD Millipore, Billerica, MA, USA). The membrane was blocked in TBS containing 0.001% Tween-20 (TBS-T) and 5% bovine serum albumin (Bovogen Biologicals Ltd., Victoria, Australia) at 4°C for 90 min. After washing, the membrane was incubated overnight at 4°C with the following primary antibodies: Rabbit anti-GAPDH (1:1,000; EMD Millipore, rabbit anti-SOD-1 (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-SOD-2 (1:1,000; Santa Cruz Biotechnology), and mouse anti-catalase (1: 1,000; Santa Cruz Biotechnology). Subsequently, membranes were washed 3 times with TBS-T for 10 min and incubated with goat anti-rabbit IgG (1:2,000; Santa Cruz Biotechnology) and goat anti-mouse IgG (1:2,000; Santa Cruz Biotechnology.) secondary antibody conjugated to alkaline phosphatase for 1 hr at room temperature. The membrane was washed 3 times with TBS-T for 10 min. Membranes were exposed to Luminata (EMD Millipore) and protein bands were imaged using a Kodak Image Station 440CF (Kodak, Rochester, NY, USA) and were quantified using Kodak ID version 3.5 densitometry software (Kodak).
Nitrite assay
The accumulation of nitrite, and indicator of the production of NO, was determined using a colorimetric assay with a Griess reagent. Hippocampal nitrite was assayed using a nitric oxide assay kit (Abcam, Cambridge, MA, USA) according to the manufacturer’s instructions. The nitrite concentration was obtained according to the standard curve generated after measuring absorbance at a wavelength of 540 nm using microplate reader (Hidex, Turku, Finland).
Statistical analysis
All data were analyzed using IBM SPSS ver. 18.0 (IBM Co., Armonk, NY, USA) by one-way analysis of variance followed by Tukey post hoc test to compare among the experimental groups. Results are presented as the mean±standard deviation. P<0.05 was considered to indicate a statistically significant difference.
RESULTS
Effects of previous exercise on the number of nNOS-positive cells in dentate gyrus
In the present results, KA group showed the number of nNOS-positive cells was increased compared with SC group (Figs. 1, 2). By contrast, in the KE group, decreased the number of nNOS-positive cells was observed in dentate gyrus compared with the KA group (Figs. 1, 2).
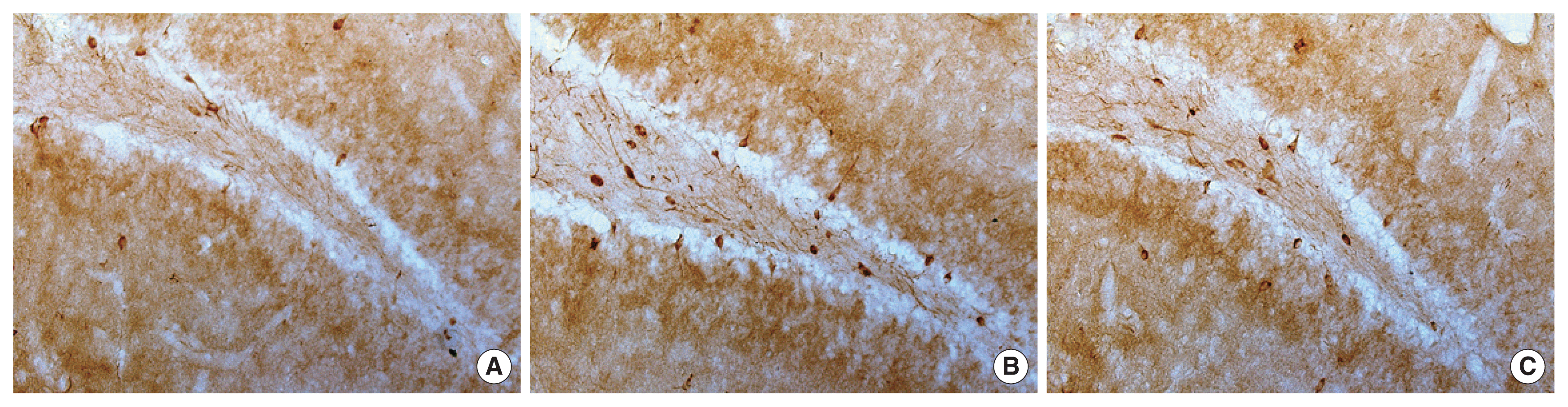
The nNOS-positive cells in dentate gyrus of SC (A), KA (B), and KE (C) group. Results are represented as the mean±standard deviation. Different letters represent significantly difference as P<0.05. nNOS, neural nitric oxide synthase; SC, saline control group; KA, kainic acid control group; KE, previous exercise and kainic acid group.

The number of nNOS-positive cells in dentate gyrus (A) and nitrite level in the hippocampus (B). Results are represented as the mean±standard deviation. Different letters represent significantly difference as P<0.05. nNOS, neuronal nitric oxide synthase; SC, saline control group; KA, kainic acid control group; KE, previous exercise and kainic acid group.
Effects of previous exercise on nitrite level in hippocampus
In the present results, KA group showed increased nitrite level in hippocampus compared with SC group (Fig. 2). By contrast, in the KE group, decreased nitrite level was observed in hippocampus compared with the KA group (Fig. 2).
Effects of previous exercise on SOD-1, SOD-2, and CAT expressions in hippocampus
In the present results, KA group showed reduced SOD-1, SOD-2, and CAT expressions in hippocampus compared with SC group (Fig. 3). By contrast, in the KE group, increased SOD-1, SOD-2, and CAT expressions were observed in hippocampus compared with the KA group (Fig. 3).

The expression of SOD-1 (A), SOD-2 (B), and CAT (C) in the hippocampus. Results are represented as the mean±standard deviation. Different letters represent significantly difference as P<0.05. SOD, superoxide dismutase; CAT, catalase; SC, saline control group; KA, kainic acid control group; KE, previous exercise and kainic acid group.
DISCUSSION
Epileptic seizure is known to accompany hippocampal neurodegeneration (Sloviter, 2005). However, no clear mechanism to cause damage of the hippocampus is known. Therefore, present study was conducted to investigate the expression of NO and changes of antioxidant enzymes in kainic acid model as well as to identify the neuroprotective effects of exercise preconditioning. As a result, the number of nNOS-positive cells and the level of nitrite were increased and expressions of SOD-1, SOD-2, and CAT were decreased by injection of kainic acid.
The animals injected kainic acid is a model similar to the temporal lobe epilepsy of humans and has been applied in many studies related to epilepsy (Sharma et al., 2007). Kainic acid model is known to induce neuronal death, particularly in the hippocampus, which is highly relevant to glutamate, an excitable neurotransmitter (Patel, 2004). The activity of kainate receptor by kainic acid has been reported as one of the major regulatory factors related to glutamate excitotoxicity, which is known to be associated with oxidative stress (Shin et al., 2011).
Excitotoxicity is an oxidative neurodegenerative response of increased NO caused by hyper-influx in cellular Ca2+ by over-activity of glutamate (Shin et al. 2011). Kainic acid also influences the overgeneration of NO by inducing an increase in cellular Ca2+ in indirect after coupling with kainate receptors. Increasing nitrite induces neuronal death in hippocampus with seizure after injection of kainic acid (Cosgrave et al., 2008; Kim et al., 2011). The study of Wang et al. (2018) suggested that nNOS was increased in hippocampus with inducing oxidative stress, which showed nNOS is involved in the production of NO by kainic acid. In this study, the results of an epileptic seizure by kainic acid showed that nitrite was increased with nNOS-positive cells in the hippocampus.
The brain is known to be highly susceptible to oxidative stress due to many aerobic metabolism and high level of polyunsaturated acid and iron load (Benatti et al., 2004). However, the human body has an antioxidant system, as defense system against oxidative stress. Antioxidant enzymes play a role to protect by reduce unstable reactive oxygen species, and the nervous system also has an antioxidant system by enzymes as SOD, CAT, and glutathione peroxidase. However, antioxidant system in the body has reduced functions in aging and in various conditions like diseases, and enzymes for antioxidant are less active in epileptic seizure (Kim et al., 2013; Shin et al., 2011). Enzyme activity of SOD and CAT was reduced in the brain after injection of kainic acid (Szaroma et al., 2012). In present study, changes in SOD-1, SOD-2, and CAT in the hippocampus showed significant reductions in the KA group, indicating a decrease in antioxidants activity due to epilepsy. This result is the simultaneous reduction of antioxidant enzymes along with the increase of NO by kainic acid injection, which indirectly shows the increase in responsiveness from oxidative stress.
Regular exercise is known to have a positive effect on the structural development of neurons and improvement of damage, and this effect continues for a certain period of time to protect the nervous system from subsequent neurodegenerative conditions (Fabel and Kempermann, 2008; Varma et al., 2016). It was reported that oxidative stress was also improved in addition to the defensive effect by increasing the activation of antioxidant enzymes (Vanzella et al., 2017).
The study of Kim et al. (2011) applied exercise after inducing epileptic seizure showed exercise is effective for epileptic seizure by the results that exercise improved the nitrite level in hippocampus. Based on the effects of exercise, in this study, the protective effects of preconditioning exercise on increased nNOS and nitrite caused by epileptic seizure were significantly improved in KE group compared to those of the KA group. Also, SOD-1, SOD-2, and CAT reduced through kainic acid were significantly improved in KE group. These results were consistent with those of Kim et al. (2013), who observed changes in antioxidant enzymes through swimming exercise in kainic acid animal model, and were shown to have decreased reactivity of oxidative stress through improvement of enzymes.
Taken together, preconditioning exercise is seen to play a neuroprotective role in oxidative stress by epileptic seizure through the suppression of NO and activation of antioxidant enzymes. Exercise has effectiveness method to prevent an oxidative stress from epilepsy.
ACKNOWLEDGMENTS
This research was supported by the Tongmyong University Research Grants 2016 (2016A033).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
