Preischemic treadmill exercise improves short-term memory by inhibiting hypoperfusion-induced disruption of blood-brain barrier after bilateral common carotid arteries occlusion
Article information
Abstract
Bilateral common carotid arteries occlusion (BCCAO) causes an abrupt reduction of cerebral blood flow, and this method has been used to investigate the effects of chronic cerebral hypoperfusion on vascular dementia and neuronal injuries. Chronic cerebral hypoperfusion leads to functional changes in the hippocampus and then results in a cognitive impairment. We investigated the effect of preischemic treadmill exercise on short-term memory and blood-brain barrier integration following cerebral hypoperfusion caused by BCCAO. The rats in the preischemic treadmill exercise and BCCAO group were made to run on a treadmill for 30 min once a day for 4 weeks. At 4 weeks after performing treadmill exercise, right carotid artery was ligated, and 1 week after, left common carotid artery was ligated. At 20 days after BCCAO, short-term memory was evaluated. Half of the rats were sacrificed 2 days after BCCAO and the other rats were sacrificed at 3 weeks after BCCAO. Immunohistochemistry and western blot were performed. Preischemic treadmill exercise alleviated impairment of short-term memory in the step-down avoidance task. Preischemic treadmill exercise reduced microvascular injury in the hippocampus. Preischemic treadmill exercise prevented the reduction of zonula occludens-1 in the hippocampus and inhibited the activation of matrix metalloproteinase-9. Therefore, pre-conditioning treadmill exercise might be used as a therapeutic strategy for the prevention of stroke in patients.
INTRODUCTION
Cerebrovascular disease is a common cause of cognitive impairment and dementia, and this disease contributes to cognitive decline in the neurodegenerative dementia (O’Brien et al., 2003). There is a correlation between the severity of memory dysfunction and the decline of cerebral blood flow (Snyder et al., 2015). Bilateral common carotid arteries occlusion (BCCAO) causes an abrupt reduction of cerebral blood flow, and this method has been used to investigate the effects of chronic cerebral hypoperfusion on vascular dementia and neuronal injuries (Farkas et al., 2007). Chronic cerebral hypoperfusion leads to functional changes in the hippocampus and then results in a cognitive impairment (Farkas et al., 2007). BCCAO model showed short-term and long-term memory impairments in the Morris water maze test, eight-arm radial maze test, and passive avoidance test (Kumaran et al., 2008; Lee et al., 2017; Zhang et al., 2011).
The blood-brain barrier (BBB) is formed by the endothelial cells of cerebral microvessels, providing an interface between the peripheral circulation and the central nervous system. The BBB is composed of brain capillary endothelial cells with pericytes, the basal lamina, and end-feet of astrocytes. In the endothelial cells, zonula occludens (ZO) family and the actin cytoskeleton are involved in regulation of cytoplasmic tight junction integrity. ZO-1 is a well-known tight junction protein and ZO-1 reflects the severity of pathological change of BBB. Chronic hypoxic hypoperfusion decreases integrity of tight junction in the BBB, and then induces increased paracellular permeability, directly contributing to cerebral vasogenic edema, hemorrhagic transformation, and increased mortality (Sandoval and Witt, 2008). Under ischemic insults, the expressions of ZO-1 and occludin in the ischemic penumbra were decreased (Xia et al., 2013).
Matrix metalloproteinases (MMPs) are extracellular proteolytic enzymes. MMPs digest the endothelial basal lamina and play an important role in degradation of the BBB. MMP-9 level in the BBB was upregulated after onset of focal cerebral ischemia, in contrast, MMP-9 knock-out mice were protected against ischemic and traumatic brain injury (Asahi et al., 2001). Tissue plasminogen activator (tPA) upregulated brain MMP-9 level in ischemic stroke, suggesting that combination therapy targeting MMPs may improve tPA therapy (Tsuji et al., 2005). Impaired tight junction proteins, ZO-1, claudin-5, and occludin, increased BBB permeability with up-regulation of MMP-2 and MMP-9 (Feng et al., 2011).
Physical exercise is known to exerts protective effect on neuronal injury and improve memory in neurodegenerative diseases (Lee et al., 2017; Lee et al., 2018a; Sim et al., 2004). However, many questions have been raised as to whether exercise before onset of cerebral stoke is also effective. Preischemic exercise ameliorated BBB dysfunction after stroke by reducing MMP-9 overexpression strengthening basal lamina (Guo et al., 2008). Cechetti et al. (2012) examined the effects of three different exercise protocols (preischemia exercise, postischemia exercise, and preischemia with postischemia exercise) against BCCAO-induced cognitive deficit. This cognitive deficit was reversed in the rats performing postischemia exercise and in the rats performing pre-ischemia with postischemia exercise (Cechetti et al., 2012). Preischemic treadmill exercise also exerted neuroprotective effect against transient cerebral ischemia by inhibiting apoptosis in the hippocampus (Aboutaleb et al., 2015). In the present study, we investigated the effect of preischemic treadmill exercise on short-term memory and BBB integration following cerebral hypoperfusion caused by BCCAO.
MATERIALS AND METHODS
Experimental animals
Male Wistar rats (80±10 g, 4 weeks old) were used in this experiment. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The rats were randomly divided into three groups (n=20 in each group): the sham group, the BCCAO group, and the preischemic treadmill exercise and BCCAO group.
Treadmill exercise protocol
The rats in the preischemic treadmill exercise and BCCAO group were made to run on a treadmill for 30 min once a day for 4 weeks starting 4 weeks after birth, according to the previously described method (Aboutaleb et al., 2015). The treadmill exercise load consisted of running at 2 m/min for the first of 5 min, at 3 m/min for the next 5 min, and then at 5 m/min for the last 20 min at 0 degree of inclination.
BCCAO
At 4 weeks after performing treadmill exercise, the rats were anesthetized by subcutaneous injection of 200 mg/kg body weight Zoletil 50 (Virbac Laboratories, Carros, France). A right ventral incision in the neck was performed to expose the right common carotid artery. The right carotid artery was double ligated with 3-0 silk (Ailee, Seoul, Korea) just below the carotid bifurcation. The left common carotid artery occlusion was performed in the same way at one week after right common carotid artery occlusion. Sham animals underwent the same operation without arterial ligation.
Step-down avoidance test
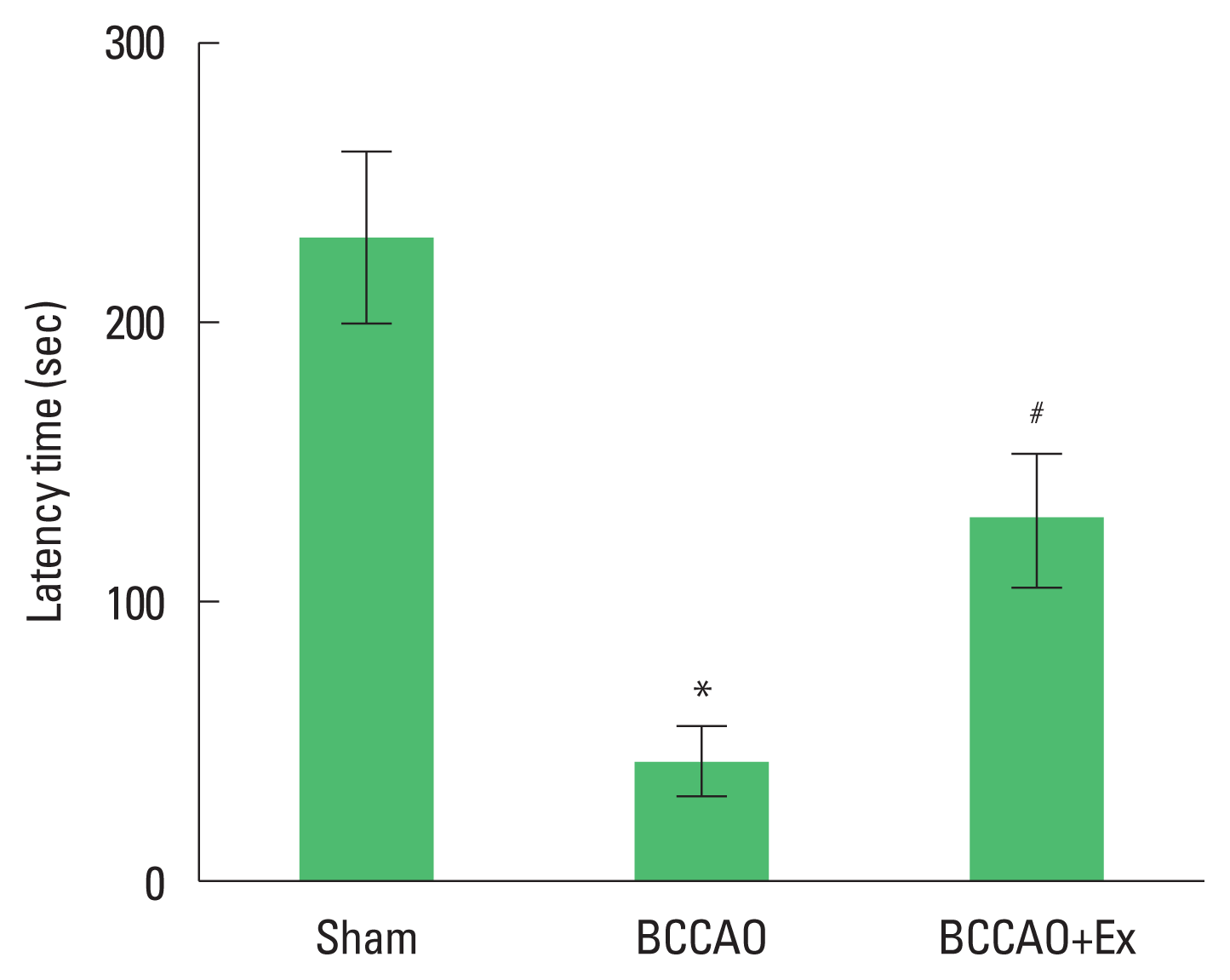
At 20 days after BCCAO, short-term memory was evaluated using the step-down avoidance test (Cho et al., 2018). The rats were positioned on a 7×25-cm platform, at the height of 2.5 cm, and allowed to rest on the platform for 2 min. The platform faced a 42×25-cm grid of parallel 0.1-cm caliber stainless steel bars, which were spaced 1 cm apart. In the training session, the animals received a 0.5-mA scramble foot shock for 3 sec immediately upon stepping down. The retention time was assessed 24 hr after the training session. The time between being placed on the platform and stepping down and placing all four paws on the grid, was defined as the latency in the step-down avoidance task. If this latency was over 300 sec, it was recorded as 300 sec.
Immunohistochemistry
Half of the rats were sacrificed 2 days after BCCAO and the other rats were sacrificed at 3 weeks after BCCAO. Immunohistochemistry was performed, in a similar way to the previous method (Moon et al., 2018). Serial coronal sections of 40-μm thickness were obtained using a freezing microtome (Leica, Nussloch, Germany). For the visualization of rat endothelial cells antigen-1 (RECA1) immunohistochemistry was performed. After being blocked with 10% normal rabbit serum for 1 hr, the sections were incubated overnight at 4°C with RECA1 antibody (1:1,000; Abcam, Cambridge, UK). The sections were then incubated for 2 hr with the biotinylated rat secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The bound secondary antibody was then amplified using a Vector Elite ABC kit (Vector Laboratories) for 1 hr at room temperature. For staining, the sections were incubated in a solution consisting of 0.02% DAB and 0.03% H2O2 in 50 mM Tris-HCl (pH, 7.6) for approximately 5 min, following which they were washed with PBS and mounted onto gelatin-coated slides. Cover slips were mounted using Permount (Thermo Fisher Scientific Inc., Waltham, MA, USA). The number of positive cells per section was counted in 5 random fields from every specimen with a Nikon Eclipse 80i microscope (magnification, Nikon Corp., Tokyo, Japan).
Western blot analysis
Half of the rats were sacrificed 2 days after BCCAO and the other rats were sacrificed at 3 weeks after BCCAO. Western blotting was performed, in a similar way to the previous method (Cho et al., 2018; Moon et al., 2018). Tissue samples harvested from the hippocampus were lysed in the protein lysis buffer containing 50 mM Tris-HCI (pH, 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% nonidet-P40 (NP40), 0.1% sodium dodecyl sulfate (SDS), 1 mM phenylmethylsulfonyl fluoride, and 100-μm/mL leupeptin. Protein concentration was measured using a colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein of 30 μg was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane (Schleicher & Schuell GmbH, Dassel, Germany). The membranes were blocked in 5% nonfat skim milk in TBST for 1 hr at room temperature followed by incubation with antibodies against ZO-1 (1:50; LifeSpan BioSciences, Inc., Seattle, WA, USA) and MMP-9 (1:1,000; Bioworld Technology, St. Louis Park, MN, USA). The primary antibodies were detected with horseradish peroxidase-conjugated secondary antibody (1:2,000; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Band detection was performed using an enhanced ECL detection system (Amersham Pharmacia Biotechnology GmbH, Freiburg, Germany). To compare the relative expressions of proteins, the detected bands were calculated densitometrically using Image-Pro Plus software (Media Cybernetics Inc., Silver Spring, MD, USA).
Statistical analysis
The results are expressed as mean±standard error of the mean. IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis. For the comparison among the groups, One-way analysis of variance and Duncan post hoc test were performed with P<0.05 as an indication of statistical significance.
RESULTS
Preischemic treadmill exercise alleviates short-term memory impairment after BCCAO
Short-term memory was measured using the step-down avoidance test (Fig. 1) Latency time in the BCCAO group was shorter compared to the sham group. In contrast, treadmill exercise increased latency time in the BCCAO group.
Preischemic treadmill exercise prevents damage to microvessels in the hippocampal CA1 region after BCCAO
Photomicrographs of RECA1-positive microvessels in the hippocampal CA1 region are presented in Fig. 2. We found that the mean perimeter of the microvessels in the hippocampal CA1 region at 48 hr after BCCAO did not differ. However, at 3 weeks postinjury, the mean perimeter of the microvessels in the hippocampal CA1 region was short length and sections were narrow and constricted diameters. In contrast, treadmill exercise alleviated microvessels damage in the hippocampal CA1 region in the BCCAO group.
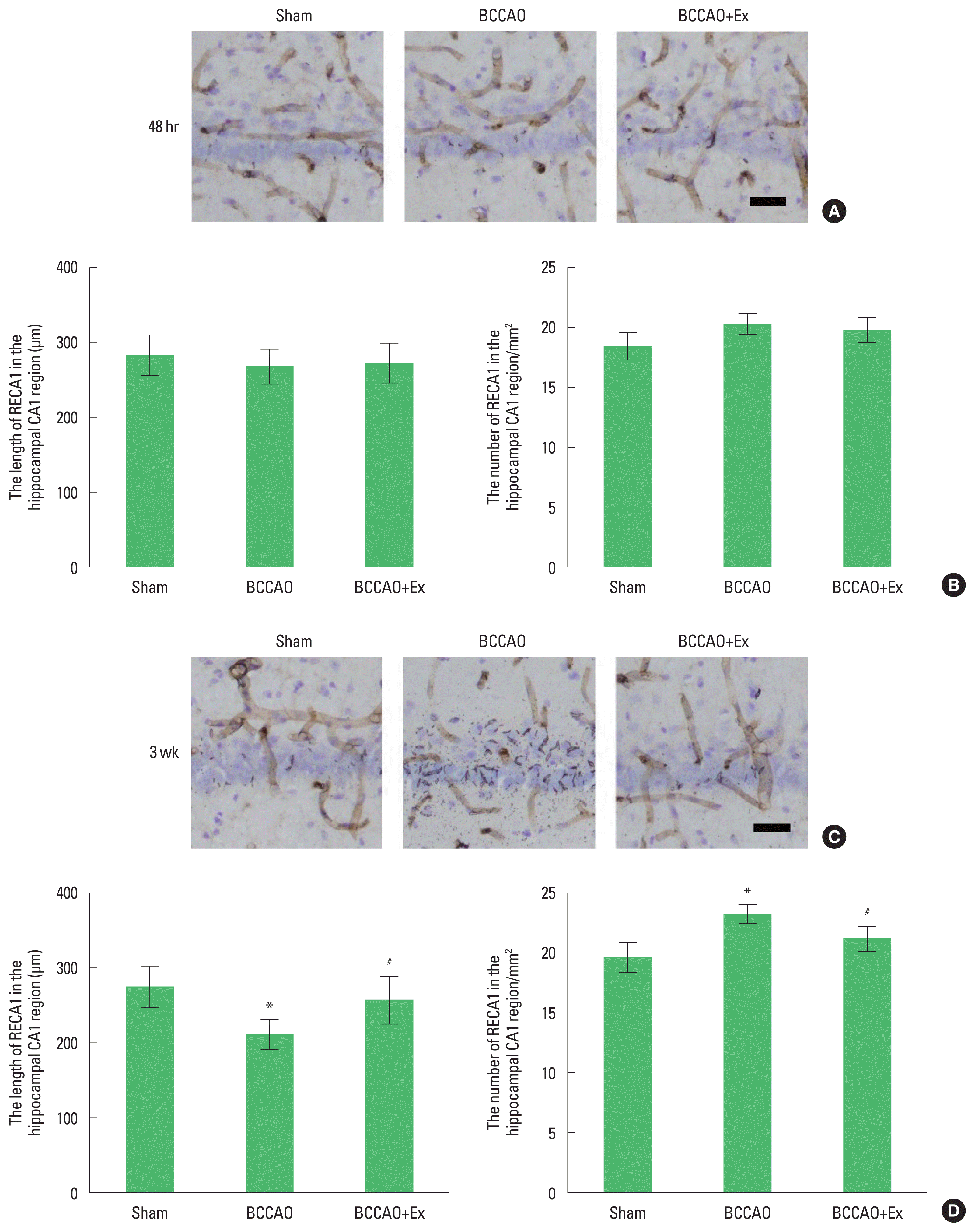
Treadmill exercise prevents damage to microvessels in the hippocampal CA1 region after bilateral common carotid artery occlusion (BCCAO). (A) Photomicrographs of rat endothelial cells antigen-1 (RECA1)-positive microvessels in the hippocampal CA1 region after 48 hr. (B) The length and number of RECA1 after 48 hr. (C) Photomicrographs of RECA1-positive microvessels in the hippocampal CA1 region after 3 weeks. (D) The length and number of RECA1 after 3 weeks. Scale bar represents 50 μm. *P<0.05 compared with the sham group. #P<0.05 compared with the BCCAO group.
Preischemic treadmill exercise prevents the loss of tight junction and inhibits activation of MMP-9 in the hippocampus after BCCAO
Presentative western blots and quantitative data are shown in Fig. 3. We found that the level of ZO-1 in the hippocampal CA1 region at 48 hr after BCCAO did not differ. However, at 3 weeks postinjury, the level of ZO-1 in the hippocampal CA1 region was decreased after BCCAO. In contrast, treadmill exercise increased the expression of ZO-1 in the hippocampal CA1 region in the BCCAO group.

Treadmill exercise prevents the loss of tight junction and activation of matrix metalloproteinase (MMP)-9 in the hippocampus after bilateral common carotid artery occlusion (BCCAO). (A) Representative bands of zonula occludens (ZO)-1 and MMP-9 in the hippocampus after 48 hr. (B) Relative expressions ZO-1 and MMP-9 after 48 hr. (C) Representative bands ZO-1 and MMP-9 in the hippocampus after 3 weeks. (D) Relative expressions ZO-1 and MMP-9 after 3 weeks. *P<0.05 compared with the sham group. #P<0.05 compared with the BCCAO group.
We found that the level of MM-9 in the hippocampal CA1 region at 48 hr after BCCAO did not differ. The level of MMP-9 in the hippocampal CA1 region was increased after BCCAO. In contrast, treadmill exercise decreased the expression of MMP-9 in the hippocampal CA1 region in the BCCAO group.
DISCUSSION
Learning and memory are impaired following chronic cerebral hypoperfusion induced by BCCAO in rats (Cechetti et al., 2012; Farkas et al., 2007). Treadmill exercise significantly ameliorated the spatial navigation performance of rats in the Morris water maze following chronic cerebral hypoperfusion (Lee et al., 2018b). The results of the present study demonstrate that preischemic treadmill exercise may attenuate short-term memory disturbance following chronic cerebral hypoperfusion.
Cerebral ischemia causes degradation of the basal lamina of microvessels and produces proteases known to degrade the extracellular matrix constituents (Fukuda et al., 2004). Cerebral hypoperfusion induced microvascular injury in the cortex and hippocampus, in contrast, treadmill exercise improved microvascular damage (Lee et al., 2017). The results of the present study demonstrated that preischemic treadmill exercise prevents damage to microvessels in the hippocampal CA1 region at 3 weeks after BCCAO. These results indicate that preischemic treadmill exercise prevents microvascular injury in the hippocampus following chronic cerebral hypoperfusion.
Cerebral ischemia is a major contributor to BBB disruption via tight junction degradation (Liu et al., 2009; Yang et al., 2007). Early exercise significantly inhibited the ischemia-induced reduction of tight junction (Zhang et al., 2013). Treadmill exercise attenuated the loss of ZO-1 in the brain after BCCAO (Lee et al., 2017). In the present study, at 3 weeks post-injury, the level of ZO-1 in the hippocampus was decreased after BCCAO. In contrast, preischemic treadmill exercise increased the expression of ZO-1 in the hippocampus after BCCAO. These findings suggest that preischemic treadmill exercise may offer protective effects on BBB disruption induced by chronic cerebral hypoperfusion.
Degradation of tight junction of the BBB is related to an increase in MMPs activity. Following transient focal ischemia, MMP-9 knock-out mice significantly reduced BBB disruption, which correlated with the degradation of ZO-1 compared to wild-type mice (Asahi et al., 2001). Preischemic exercise ameliorates BBB dysfunction in stroke by reducing MMP-9 expression (Guo et al., 2008). Treadmill exercise ameliorated the overexpression of MMP-9 after BCCAO (Lee et al., 2017). In the present study, preischemic treadmill exercise could significantly suppress the activation of MMP-9 in the brain following chronic cerebral hypoperfusion. Based on these findings, our results indicate that preischemic treadmill exercise may provide protective effects on BBB disruption from overexpression of MMP-9 induced by chronic cerebral hypoperfusion.
Preischemic treadmill exercise may ameliorate shot-term memory impairment and prevent microvascular injury in the hippocampus following chronic cerebral hypoperfusion. Therefore, preconditioning treadmill exercise might be used as a therapeutic strategy for the prevention of stroke in patients.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This study was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2016S1A5B5A01022873).