Effects of two intensities of treadmill exercise on neuromuscular recovery after median nerve crush injury in Wistar rats
Article information
Abstract
Considering the potential action of exercise on neuroplasticity and the need to adapt protocols to enhance functional recovery after nerve injury, this study evaluated the effects of two intensities of treadmill exercise on nervous and muscular tissues and functional recovery after nerve crush injury. Wistar rats were distributed into sedentary group (SED), and 10 m/min (EG10) and 17 m/min (EG17) exercise groups. The exercise started one week after the injury. Ten daily sessions were performed with a 2-day interval after the fifth day. The flexor digitorum muscle and two segments of the median nerve were analysed histomorphometrically by light microscopy and computer analysis. Function was evaluated by grasping test, in 3 moments. Approval number: 016/2013. In the proximal segments of the median nerve, the diameter of myelinated fibres and axon, the myelin sheath thickness and the ratio of axon diameter to fibre diameter (g ratio) were significantly larger (P<0.05) in the EG10. The number of myelinated fibres was lesser in the EG17 than the other groups (P<0.05). No difference in the number of myelinated fibres among groups was observed in the distal segments, but the SED presented significantly larger axon and fibre diameters than those that performed exercise. The EG10 presented greater area and diameter of muscle fibres (P<0.05) and functional improvement observed on the 21st day after injury (P<0.05) compared with the EG17 and SED. Continuous exercise at 10 m/min accentuates nerve regeneration, accelerating functional recovery and preventing muscle atrophy.
INTRODUCTION
Peripheral nerve injury is a substantial clinical problem that has devastating consequences for patients (Panagopoulos et al., 2017; Sullivan et al., 2016) and poses a challenge to rehabilitation teams (Faroni et al., 2015; Faturi et al., 2016). Lesions of the peripheral nerves occur most frequently in the upper extremities, with the ulnar and median nerves being the most often affected (Daneyemez et al., 2005; Kouyoumdjian et al., 2017; Miranda and Torres, 2016; Szyłejko et al., 2015); and compression being the most common mechanism (Taylor et al., 2008). Most daily activities require functional hands; therefore, nerve injuries in the upper extremities can cause substantial problems for individuals of all ages (Daneyemez et al., 2005; Miranda and Torres, 2016; Szyłejko et al., 2015).
Nerves can be damaged to varying degrees and by many causes (Daneyemez et al., 2005; Kouyoumdjian et al., 2017). The ultimate goal of peripheral nerve repair is effective recovery of function (Udina et al., 2011b); however, this is almost never complete (Geuna et al., 2016; Gordon and Borschel, 2017). After an injury, a peripheral nerve regenerates spontaneously at a very slow rate, approximately 1 mm per day, depending on the lesion site (Sulaiman and Gordon, 2013; Sullivan et al., 2016). Moreover, the regeneration capacity of the peripheral nervous system (PNS) decreases over time (Boerboom et al., 2017).
Some neurorehabilitation strategies have shown benefits in terms of improved functional recovery after PNS injuries. Treatments using exercise have been increasingly studied due to their positive results in the rehabilitation of PNS injuries and their role in neuroprotection and neuroregeneration (Armada-da-Silva et al., 2013; Cobianchi et al., 2017). Different exercise types, such as resistance training with weight (50 to 250 g) attached to the animals’ tails (Ilha et al., 2008), swimming exercise (20 to 40 min) with a progressive load of up to 10% body weight (Coradini et al., 2015), passive cycling of the limbs (Udina et al., 2011b) and treadmill exercises (Boeltz et al., 2013; Bonetti et al., 2017; English et al., 2011) have been tested to treat PNS injuries.
Exercise promotes axonal regeneration and functional recovery and may improve sensory-motor coordination and restoration of adequate circuitry at the spinal level (Udina et al., 2011b). Exercise also increases axon elongation (Sabatier et al., 2008), promotes Schwann cell regenerative properties and nerve repair (Gordon and English, 2016), and improves functional muscle recovery after nerve injury (Marqueste et al., 2004). For all these reasons, exercise has been attracting interest as a way of accelerating axonal growth and restoring function. However, the literature has shown conflicting evidence regarding the beneficial and deleterious effects of exercise on peripheral nerve regeneration and muscle reinnervation, possibly due to variations in the type of nerve injury, the sort of exercise realized, the duration and intensity of training and when it is initiated (Armada-da-Silva et al., 2013; Cobianchi et al., 2017; Udina et al., 2011a).
Standard rehabilitative exercise protocols for nerve injury need to be properly defined. The intensity of exercise seems to be a critical parameter for neuroprotection and should be taken into consideration (Cobianchi et al., 2017). The aim of this study was to verify the effects of two intensities of treadmill exercise on median nerve regeneration after crush injury in rats. The histomorphometry of the median nerve and the histomorphometry and functionality of the flexor digitorum muscle were evaluated.
MATERIALS AND METHODS
Animals
This research was approved by the Ethics Committee on Animal Use of the Federal University of Jequitinhonha and Mucuri Valleys, protocol number 016/2013. The experiments were performed on 24 female Wistar rats (7 weeks old; approximately 170 g). The animals were housed under controlled environmental conditions (temperature 22°C±1°C and humidity 40%–50%) with a 12:12-hour light–dark cycle and free access to food (Nuvilab CR1; Nuvital Nutrientes S/A, Paraná, Brazil) and water.
Experimental groups
The animals were randomly divided into three groups with eight each. In the sedentary group (SED), rats underwent median nerve crush and were not exercised. In exercise group 10 m/min (EG10) and exercise group 17 m/min (EG17), the rats underwent median nerve crush. Seven days after the injury, the animals performed one hr of continuous treadmill exercise training at velocities of 10 m/min and 17 m/min, respectively, 5 days per week, for 2 weeks.
The animals performed the exercise on a motorized treadmill (Insight EP-131; Ribeirão Preto, Brazil) with 6 individual bays, each measuring approximately 10 cm in width and 38 cm in length. The exercises were performed with supervision, and no additional stimuli of any kind were applied during the exercise. The rats underwent one week of adaptation before the nerve crush surgery. They walked daily on the treadmill for 5 days per week at a velocity of 10 m/min (EG10) and 17 m/min (EG17). The training was performed for 10 min on the first day, 20 min on the second day, 30 min on the third day, 45 min on the fourth day and 60 min on the fifth day.
Surgical procedures for nerve injury
For the surgical procedures, the animals were anaesthetized by intraperitoneal injection of ketamine (10%) and xylazine (2%) (0.1 mL/100 g – Sespo Ind. Com. Ltda, São Paulo, Brazil). After hair trimming, the right median nerve was exposed 10 mm above the elbow under aseptic conditions, and a crush injury was induced for 2 min by using a standard haemostatic forceps closed to the second notch (Santos et al., 2012). The location of the crush injury was identified with a point on an adjacent muscle. The skin was closed with 4-0 silk sutures. After surgery, digluconate chlorhexidine (Merthiolate; Gold Lab, São Paulo, Brazil) was applied and the animals were kept in individual cages until they awoke. For the first 3 days, paracetamol (750 mg/L) was added to the water for pain reduction.
Functional evaluation
The recovery of median nerve function was assessed by means of the grasping test (Bertelli and Mira, 1995). Briefly, the rats were gently lifted by the tail and allowed to grasp a grid connected to an ordinary electronic balance. While grasping the grid, the animal continued to be lifted by the tail with increasing force until it lost its grip. At this precise moment, the value shown by the balance was recorded. Before surgery, the right median nerve function was assessed in all the animals in order to obtain baseline control values (presurgery). The animals were then retested on postoperative day 11 and at the end of the experiment (day 21). The contralateral forepaw was temporarily wrapped with adhesive tape to prevent it from grasping the grid. All the tests were performed by a single, skilled investigator who was blinded to the experimental group to which each animal belonged.
Nerve and muscle histological and morphometric analysis
Twenty-one days after the injury, the animals were weighed and anaesthetized. The median nerve (proximal segment – site of crush and distal segment – forearm, distal 1/3) and the flexor digitorum muscle were exposed, fixed in situ with 4% paraformaldehyde and removed.
After removal, the nerves were kept in the same fixative solution for an additional 24 hr, postfixed with 2% OsO4 for 12 hr, and processed for paraffin embedding. After careful positioning of the nerve fragments in embedding molds, transverse sections were cut at 5 μm. The sections were observed and photomicrographed on an optical microscope (Primo Star – ZEISS, Jena, Germany) coupled to an Axiocam ERc 5s camera (ZEISS) and were analysed in the image processing program ImageJ. The area of the fascicles was measured manually, and the number of capillaries was counted. Analysis of minimal diameters of axons and myelinated fibres, myelin sheath thickness and the ratio of axon diameter to fibre diameter (g ratio) were carried out in 30% of the digitized image of each specimen obtained through the 100× objective (Santos et al., 2007); this process was conducted in a randomized and blind manner. Transverse sections of the flexor digitorum muscle were obtained after the process of paraffin embedding and were stained with hematoxylin-eosin. The minimal Feret diameter and cross-sectional area of 200 random fibres were analysed (Gigo-Benato et al., 2010; Russo et al., 2008).
Statistical analysis
The data was analysed by using the software GraphPad Prism, ver. 5.0 (GraphPad Software Inc., San Diego, CA, USA). The normality of all data distributions was checked with the Shapiro–Wilk test. Normally distributed data was compared by analysis of variance (ANOVA), followed by Tukey test; data without a normal distribution was compared by Kruskal–Wallis test followed by Dunn test. Paired t-test was used for the functional results pre and postoperative within the same group. Differences were considered significant at P<0.05.
RESULTS
Photomicrographs of the proximal (i.e., injury site) and distal segments of the median nerve in the three groups studied are presented in Fig. 1. The proximal segments of the studied groups presented endoneural areas with myelin fibres of various diameters and dispersed capillaries; a morphological similarity between SED and EG10 was observed, while EG17 presented a reduced number of myelin fibres. Small-diameter myelin fibres with enlarged endoneural connective tissue space in the distal segments of the three groups analysed were observed.

Digitized images of transverse semi-thin sections of the regenerated median nerve 21 days after nerve crush lesion. The proximal segment (first column) and distal segment (second column) of the median nerve in SED (A and D), EG10 (B and E), and EG17 (C and F) rats are shown. Magnified by 1,000 times. Scale bars=20 μm (A to F). SED, sedentary group; EG10, exercise group 10 m/min; EG17, exercise group 17 m/min.
The morphometric data obtained from the proximal and distal segments of the median nerve of the three groups is presented in Table 1. In the proximal segments of the median nerve, the myelinated fibres diameter, the axon diameter, the myelin sheath thickness and the g ratio were significantly larger in the EG10 group than in the other groups. The number of myelinated fibres was lesser in the EG17 than the other groups, but was observed an improvement in the diameter of myelin fibres and axons in relation to the SED group. No difference in the number of myelinated fibres among groups was observed in the distal segments, but the SED group presented significantly larger axon and fibre diameters than those that performed exercise. No significant difference in the number of capillaries endoneural was observed between the groups. The values for SED, EG10, and EG17 in the proximal segment were 11±8, 8±6, and 6±1, respectively; and 10±8, 10±8, and 7±5 in the distal segment, respectively.

Morphometric data of the myelinated fibres for proximal and distal segments of the median nerve in the three groups studied
The distribution histograms of myelin fibres and axons of the proximal segment of the median nerve presented peak values of 3 μm for myelinated fibre diameter and 2 μm for axon diameter. A discrete rightward shift in the EG10 group was observed, but was not repeated in the distal segment. The peaks found in the distal segments were also similar between the groups, from 1.5 to 2 μm for the myelin fibre diameter and 1 μm for the axon (Fig. 2).

Histograms showing the distribution of myelinated fibre (A and C) and axon (B and D) diameters for the proximal (A, B) and distal (C, D) segments of the median nerve. SED, sedentary group; EG10, exercise group 10 m/min; EG17, exercise group 17 m/min.
The morphological characteristics of the fibres of the flexor digitorum are shown in Fig. 3. All groups maintained the polygonal shape of the muscle fibres; although, EG10 presented greater calibre of muscle fibres and fewer connective tissue between the cells than the other groups. On the other hand, EG17 presented reduced muscle fibre size and a large number of muscle fibres per image field.
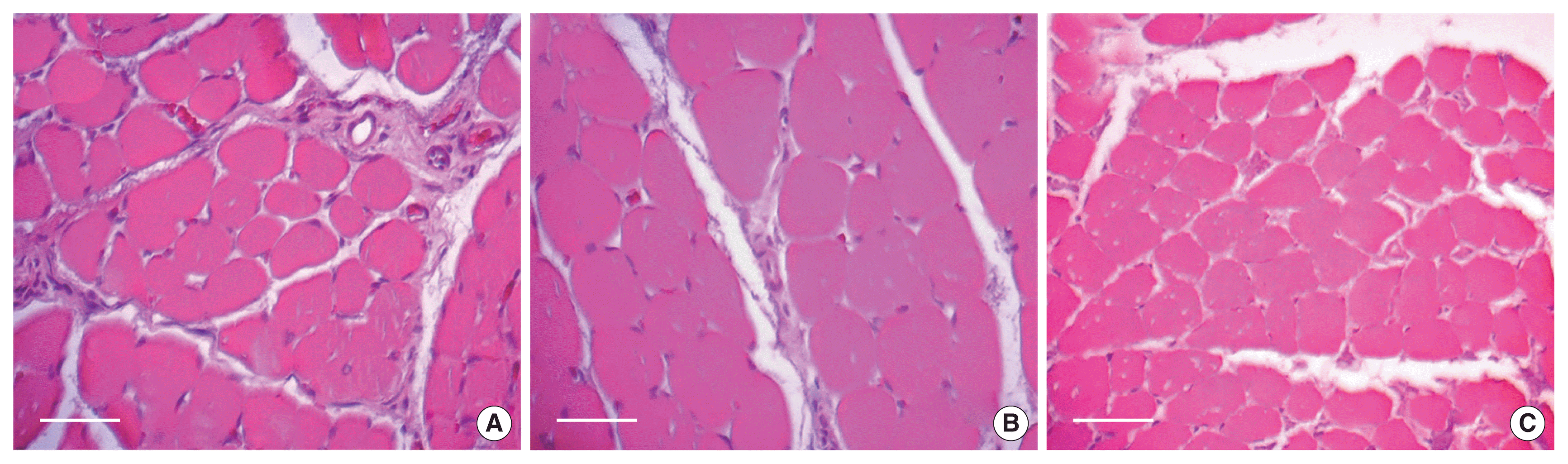
Digitized images of transverse semi-thin sections of the flexor digitorum muscle on the 21st day after injury. (A) SED. (B) EG10. (C) EG17. Magnified by 400 times. Scale bars=40 μm (A to C). The sections were stained with hematoxylin-eosin. SED, sedentary group; EG10, exercise group 10 m/min; EG17, exercise group 17 m/min.
The morphometric data obtained from the flexor digitorum muscle fibres of the three groups is presented in Fig. 4. The EG10 presented greater area and diameter of muscle fibres compared with the SED and EG17 groups (P<0.05).
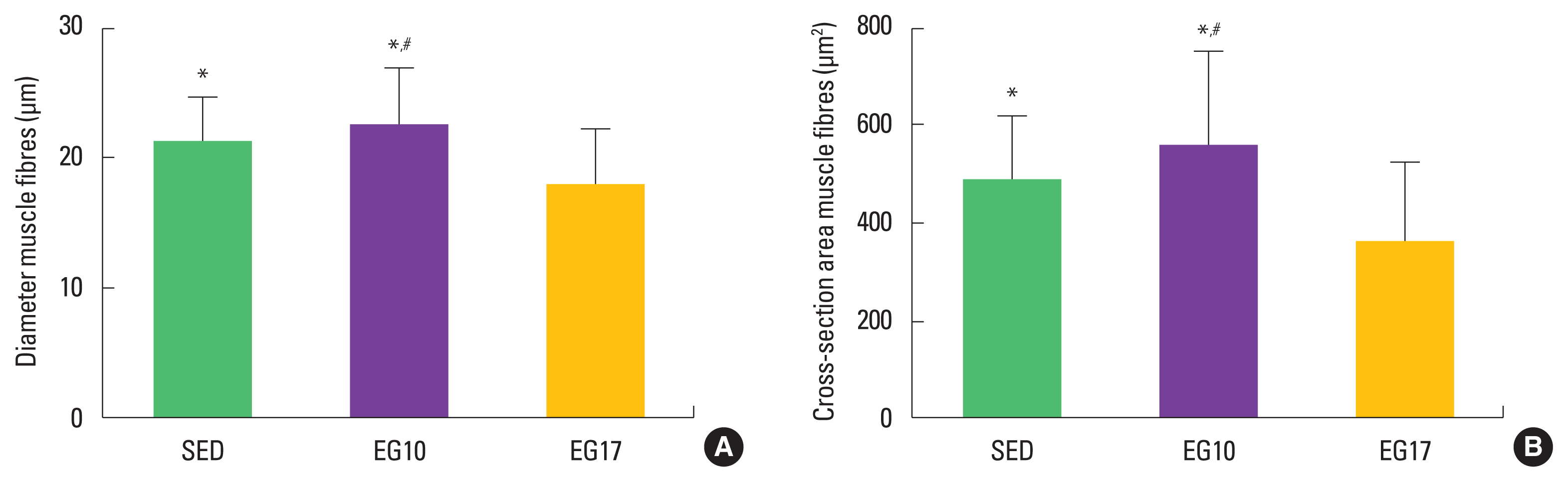
Mean values of the flexor digitorum muscle. (A) Diameter of the cross section of the muscle fibre. (B) Cross-sectional area of muscle fibre. SED, sedentary group; EG10, exercise group 10 m/min; EG17, exercise group 17 m/min. *Differs from EG17 at P<0.05. #Differs from SED at P<0.05.
The results of the functional assessment are reported in Fig. 5. On the 11th day after injury, the groups that performed exercise presented greater muscular strength than the sedentary group. The strength at the 11th day corresponded to 76% (EG10), 54% (EG17), and 28% (SED) of the strength on the preoperative day. A significant difference (P<0.05) between the EG10 compared with the EG17 and SED groups was observed on the 21st day after injury, and the values obtained were 136%, 94%, and 109% of the values on the preoperative day, respectively. EG10 presented better results (P=0.002) on day 21 than preoperative (295±48 g and 217±35 g, respectively). On the other hand, no difference between EG17 and SED groups in the preoperative day and 21 postoperative day was found.
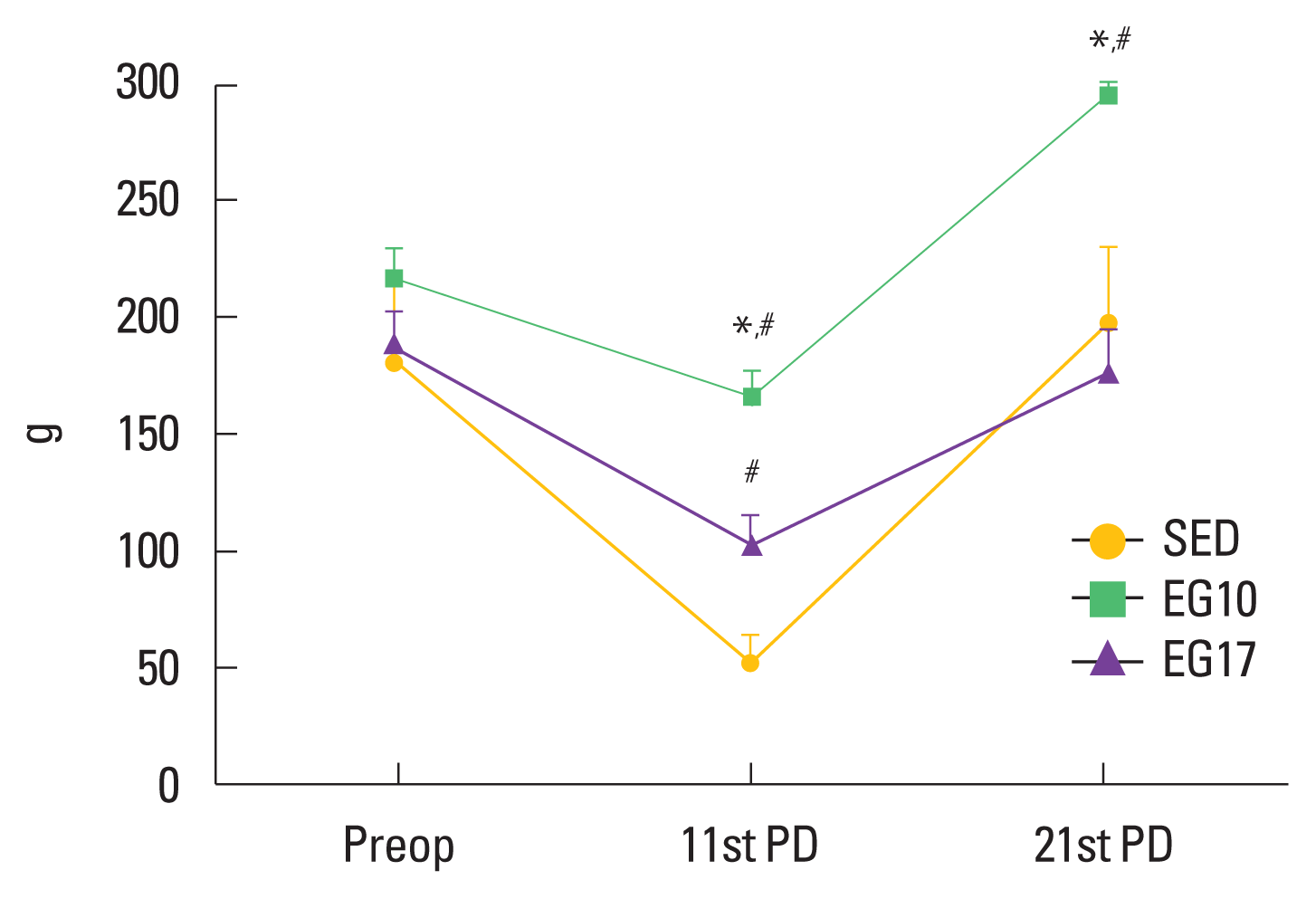
Comparison of grip strength between the groups. Data is presented as the mean±standard error of the mean. Differences were considered significant at P<0.05. Preop, preoperative day; PD, postoperative day; SED, sedentary group; EG10, exercise group 10 m/min; EG17, exercise group 17 m/min. *Indicates a significant difference from EG17. #Indicates a significant difference from SED.
DISCUSSION
Peripheral nerve injury results in severe disability, pain and decreased quality of life (Panagopoulos et al., 2017; Sullivan et al., 2016); therefore, methods that facilitate the process of adequate nerve regeneration and functional return, such as therapeutic exercises, are of great relevance. In the upper limbs the distance to the target organs is shorter than in the lower limbs, decreasing the time required for reinnervation and thereby presenting an experimental advantage (Bontioti et al., 2003). Forelimb models have no contracture or autotomy that interferes in functional evaluation (Bertelli and Mira, 1995; Bontioti et al., 2003). Also, the grasping test is useful since the median nerve and finger flexor function can be easily assessed, furthermore, the grasping test is an objective and reliable method that allows researchers to track recovery progress during the repair process (Bertelli and Mira, 1995; Papalia et al., 2003, 2016; Ronchi et al., 2009).
In this study, crush injury of the rat median nerve was used as a model to investigate the hypothesis that two treadmill training intensities would produce different effects on functional recovery and on histological and morphometric aspects of the median nerve and flexor digitorum muscle. Crush lesions are simple, have lower variability in the regeneration response than transections and is a good model for studding motor recovery (Ronchi et al., 2009).
The treadmill training at 10 m/min resulted in an improvement in the variables related to the histomorphometry of the flexor digitorum muscle and in the variables related to myelinated fibres proximally to lesion site. No improvement in the variables related to the myelinated fibres was observed in the distal segment of the median nerve, however, the g ratio—a ratio that is relevant to ideal saltatory conduction and useful for assessing nerve diseases (a ratio of approximately 0.6 is ideal) (Gutrecht and Dyck, 1970; Rushton, 1951)—was similar in this segment to that of the sedentary group and more favourable than that of the 17 m/min group. Better g ratio and higher thickness of myelin sheath were also found in the study of Ilha et al. (2008) 5 mm distal to the crushing site in the trained group (treadmill 9 m/min).
The distributions of the myelinated diameters were unimodal in all groups, as found in the corresponding nerve segments in uninjured animals of the same age (Santos et al., 2007), but with lower peaks. In the proximal segment, the 10 m/min group presented the closest distribution to that found in normal control data (Santos et al., 2007).
The angiogenic action of exercise is known (Lansford et al., 2016; Terra et al., 2012); a higher number of vessels could minimize the effects of hypoxia and enhance nerve regeneration (Kakihata et al., 2016). In this study, no difference in the number of endoneural vessels was observed among groups, in contrast to what was found in a sciatic nerve crush model, in which exercise promoted increase in endoneural vessels (Kakihata et al., 2016).
A fast restoration of function seen in this study for both exercise intensities, as assessed on the eleventh postinjury day, corroborated with the results of a prior study that applied 5 weeks of training at an intensity of 9 m/min for 60 min in a sciatic nerve crush model. In that study, an improvement in the nerve function index was observed after one week of training, in contrast to the sedentary group (Ilha et al., 2008). In this study, the intensity training of 17 m/min accelerated the functional restoration but did not maintain it as the intensity of 10 m/min did; these functional results are associated with the histomorphometric findings of the muscle fibres in the 17 m/min group, which show that, over time, this protocol can be harmful in the recovery of muscle and nervous tissues and function.
A study (Boeltz et al., 2013) with an exercise protocol of 10 m/min, starting the training on the third day after the injury, was performed to verify functional restoration after the repair of sciatic neurotmesis with fibrin glue. They found that moderate exercise enhanced functional recovery, although, there was no complete functional recovery. In another study in a sciatic neurotmesis model (Cannoy et al., 2016), after an exercise protocol of 10 m/min for 60 min in two diferente slopes 0° and 20°. The second group presented worse results in functional recovery, despite showing improvement in motor axon regeneration.
Analysis of the nerve, the muscle and their function, similar to what was conducted in this study, was performed in a mouse model of neurotmesis followed by neurorrhaphy (Park and Höke, 2014). The exercise programme consisted of 60 min of continuous running at a speed of 10 m/min. The proposed protocol resulted in faster recovery of forelimb grip function, better regeneration of the median nerve at 3–5 mm distal to the neurorrhaphy and larger fibre size in the forearm flexor digitorum muscle.
Exercise protocols with velocities close to 17 m/min were used in sciatic nerve crush models. In one study (Jang and Lee, 2015), the exercise performed at 5 m/min during 20 min for the first week and at 15 m/min during 60 min from the second to the fourth weeks, significantly increased functional recovery. In another study (Seo et al., 2006), the animals walked on a treadmill at a velocity of 18 m/min for 30 min. An improvement in axonal regeneration and function were observed in the exercised group compared with the sedentary. These results may be due to the protocols chosen; in the study of Jang and Lee (2015), the speed was slightly lower, while in the study of Seo et al. (2006) the daily exercise time of 60 min was divided into two sessions per day.
The intensity of the exercise was determined by exercise duration, slope and speed (Abreu et al., 2016; Marqueste et al., 2004). However, interval and continuous protocol training, with their different physiological adaptations (de Araujo et al., 2015), may have differential effects on the results and need to be considered. High-intensity interval exercise on treadmill was enough to promote axonal growth similar to that promoted by sixty minutes on a treadmill at 10 m/min after fibular nerve transection in mice (Sabatier et al., 2008).
Thus, it is important to consider the variables that determine the intensity of treadmill exercise rehabilitation after nerve injury and to analyse which type of protocol is most appropriate for each situation. Perhaps longer protocols require moderation in speed, as seen at the suboptimal recovery of the 17 m/min group and in the slope of the treadmill, as reported by Cannoy et al. (2016), however, higher speeds should not be discarded, since the duration of exercise can be adjusted. In addition, it is necessary to consider other specifics that may influence the results of an exercise protocol, such as modality (Bonetti et al., 2017), age (Cunha et al., 2011), gender (Acosta et al., 2017), and prior disease (Kim and Lee, 2010).
Exercise is a rehabilitation strategy is potentially favourable to nerve regeneration and functional restoration; protocols need to be investigated to maximize their effectiveness (English et al., 2011). This study evaluated the action of two exercise intensities on the regeneration of two median nerve segments, distal and proximal to the injury. Also, on the muscle and on the function restoration. The results confirmed that moderate exercise accentuates nerve regeneration, accelerates functional recovery and prevents muscle atrophy. The intensity of the exercise has a key role in the function restoration and in the muscular and nervous tissues recovery process, since an increase of 7 m/min in velocity produces undesired results. Moderate exercises should be considered as a potential therapy to promote nerve regeneration and functional recovery after peripheral nerve injury.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
The authors thank FAPEMIG (Minas Gerais State Agency for Research and Development) for financial support and scholarships. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
