Blood pressure responses after resistance exercise session in women living with human immunodeficiency virus/acquired immunodeficiency syndrome
Article information
Abstract
The aim of this study was to verify blood pressure (BP) responses after a single resistance exercise session in women with human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS). Twelve patients underwent a resisted exercise session. BP, heart rate, and rate pressure product were evaluated before and during 120 min after the session. Mean cardiovascular values before and after the session were similar (P>0.05). Analysis of the individual data revealed that for 120 min after exercise, 5 and 4 patients presented a reduction in systolic and diastolic BP of ≥4 mmHg, respectively. The clinical characteristics of the patients appear to influence BP responses after exercise. Individual data showed that some of the HIV+ women demonstrated a clinically significant decrease in BP. Although a single resistance exercise session does not decrease BP in women with HIV/AIDS, individual data present heterogeneity and individual characteristics seem to influence BP reduction after a single session of resistance exercises.
INTRODUCTION
Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) is associated with opportunistic pathologies due to low activity of the immune system (Raso et al., 2007). Highly active antiretroviral therapy (HAART) is widely recommended for patients with HIV/AIDS as a way to prevent the onset of these pathologies (Chow et al., 2017; Mendicino et al., 2016). However, HAART increases the cardiovascular risk of the patients (Hsue et al., 2009; Lipshultz et al., 2003; Zareba et al., 2005), especially women (Muyanja et al., 2016). In fact, subjects treated with HAART demonstrated increases of 4.6 mmHg in systolic blood pressure (BP) and 4.2 mmHg in diastolic BP, when compared with HIV+ patients who did not adhere to the treatment (Wilson et al., 2009).
Resistance training has been recommended for individuals living with HIV/AIDS (Grace et al., 2015), as it promotes improvements in muscle function that are highly affected by the disease. In addition to these effects, studies in other populations have shown that a single session of resistance exercise reduces BP in a broad range of conditions (Queiroz et al., 2009; Teixeira et al., 2011). These acute reductions have been considered clinically meaningful, since they predict chronic reductions in BP after a resistance training program (Tibana et al., 2015). However, whether these benefits also occur in women with HIV+ is unknown.
The purpose of this study was to investigate the effects of single session of resistance exercise on BP in women living with HIV/AIDS.
MATERIALS AND METHODS
Study design
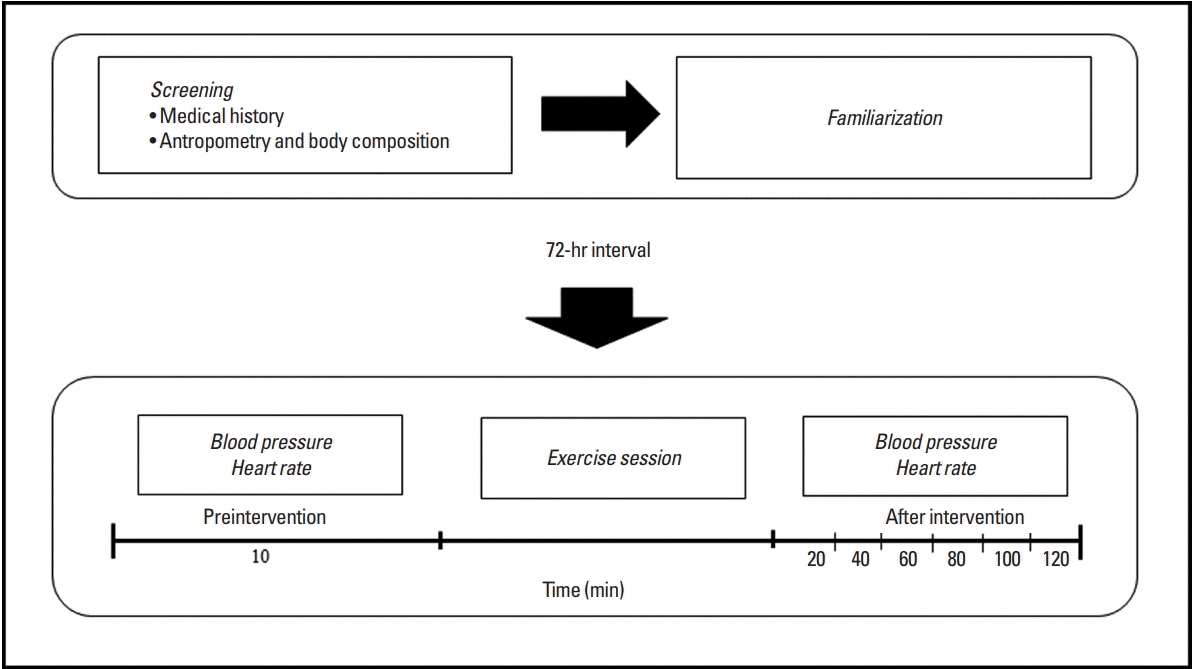
The study design is presented in Fig. 1. This study comprised two phases in which the participants performed 6 visits to the laboratory. At first, the participants performed clinical evaluation. After 24 hr, each participant underwent familiarization sessions on alternate days. Subsequently, all participants underwent a resistance exercise protocol session. Before and after the experimental session were performed cardiovascular measurements.
Subjects
Twelve sedentary women living with HIV/AIDS participated in this crossover study. Participants were included if they: (a) >18 years old, (b) had used HAART for more than 6 months; (c) were in a stable condition and their quantification of HIV viral load had been stable for the previous 6 months; (d) did not smoke; (e) had no musculoskeletal injuries that might affect their ability to perform the exercise protocol; and (f) had not used ergogenic substances in the 6 months prior to the study. The study was conducted according to ethical principles, including the Declaration of Helsinki. Ethical approval was obtained from the Ethics Committee of the State University of Maringa, Brazil (Process n° 47513115.0.0000.0104) and each participant provided written consent after familiarization with the study procedures.
Clinical data
Information on cardiovascular risk, hypertension, and HIV diagnosis were obtained from a medical history prior to the start of the study. Weight and height were obtained using a scale and a stadiometer, from which the body mass index was calculated. The waist circumference and hip circumference were registered according to international guidelines (Lohman et al., 1988). Body fat percentage was estimated by skinfold measurements according to the protocol proposed by (Jackson and Pollock, 1985).
Familiarization
All patients underwent four-familiarization sessions designed to standardize resistance exercise execution. In these sessions, they performed the following exercises: chest press, leg press 45°, lat pulldown, leg extension, triceps pulley, leg curls, and biceps scott. Each exercise was performed for three sets of 10 repetitions, with 90- and 120-sec intervals between sets and exercises, respectively. Each patient was submitted to the lowest and highest load possible in each exercise in order to perform the anchoring procedures of the OMNI resistance exercise scale (Gearhart et al., 2001). During the final familiarization session, the load corresponding to a rate of perceived exertion between five and seven on the OMNI resistance exercise scale was determined for each resistance exercise, as previously described (Lins-Filho et al., 2012).
Experimental session
After the preintervention procedures, patients performed the resistance exercise protocol. In this protocol, patients performed three sets of 10 repetitions of seven exercises (chest press, 45° leg press, lat pulldown, leg extension, triceps pulley extensions, leg curls, and biceps scott curls) with a workload of five to seven on the OMNI resistance exercise scale. Rest intervals of 90 and 120 sec were observed between the sets and exercises, respectively.
After the intervention, patients returned to the laboratory, where they remained seated for 120 min (postintervention). During this period, BP and heart rate (HR) were measured every 20 min (20, 40, 60, 80, 100, and 120).
Blood pressure
The BP was measured using a previously calibrated digital monitor (OMRON DELUXE, HEM 7200, Brazil). The cuff was adjusted to the circumference of the right arm, 2 to 3 cm above the cubital fossa at heart level. Measurements were performed in triplicate with a 1-min interval between them. All procedures followed the standardized, national recommendations for BP monitoring (Malachias et al., 2016). HR was measured using an HR monitor (Polar S810, Polar Electro Oy, Finland) and rate pressure product (RPP) was calculated by multiplying systolic BP and HR.
Statistical analysis
The Gaussian distribution of the data was verified and confirmed by the Shapiro–Wilk test. For the comparison of BP, HR, and RPP before and after exercise one-way analysis of variance for repeated measures was used. To analyze the individual BP responses after the resistance exercise sessions, we considered a reduction equal or greater than 4 mmHg (Hemmelgarn et al., 2004). The Mann–Whitney U-test was used to compare clinical characteristics among “responder” and “nonresponders” women. The level of significance was P<0.05.
RESULTS
Characteristics of the participants are presented in Table 1. The mean values of systolic and diastolic BP classified participants as normotensive.
Considering the mean values, the systolic BP, diastolic BP, HR, and RPP did not show significant changes before and after a resistance exercise session (P<0.05).
Fig. 2 shows the individual responses of systolic and diastolic BP after the resistance exercise session. Five patients presented a decrease ≥4 mmHg in systolic BP and 4 patients in diastolic BP after the exercise session.
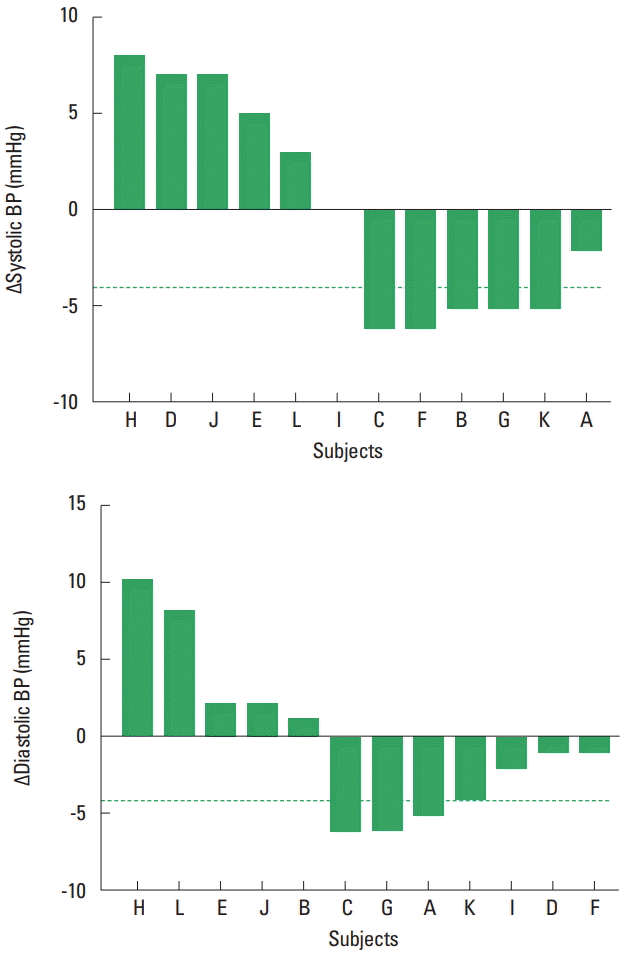
Individual responses of systolic and diastolic blood pressure (BP) after a resisted exercise session. Responders (reduction in BP in ≥4.0 mmHg; dashed line).
Table 2 shows the clinical characteristics of the patients who presented BP decreases ≥4.0 mmHg (systolic BP responders, n=5; diastolic BP responders, n=4) before and after the exercise session. Responder patients had a higher waist-hip ratio, lower time of HAART use, and lower CD4/CD8 ratio (P<0.05).
DISCUSSION
The results indicated that there was considerable variability in BP responses after a resistance exercise session among HIV+ women, since some of the subjects presented meaningful decreases in systolic or diastolic BP, while in other patients, cardiovascular parameters were not altered.
Although acute changes in BP have been widely described in the literature (Aprile et al., 2016; Queiroz et al., 2009; Teixeira et al., 2011), high variability in these responses have been reported in normotensive (Costa et al., 2016; Tibana et al., 2015), prehypertensive (Hecksteden et al., 2013), hypertensive (Kiviniemi et al., 2015; Lima et al., 2015; Moreira et al., 2016) individuals. Our findings broaden the current knowledge, showing that this variability also occurs in women with HIV/AIDS. Analysis of the individual responses demonstrated that 42% and 33% of the participants presented a clinically relevant reduction in systolic and diastolic BP, respectively. This is similar to a study in patients with peripheral artery disease (Lima et al., 2015) who demonstrated clinically significant reductions of 46% in systolic BP and 38% in diastolic BP after resistance exercise. These findings suggest that the responsiveness of BP to resistance exercise occurs in different clinical populations.
In our study, HIV+ women who presented reductions ≥4 mmHg in diastolic BP had a shorter time using HAART, a lower CD4/CD8 ratio, and higher abdominal obesity. Interestingly, these individuals present a more vulnerable immune system (CD4/CD8 ratio<1) and are more likely to present atherosclerotic plaque (Serrano-Villar et al., 2014a; Serrano-Villar et al., 2014b). Thus, our findings show for the first time that more vulnerable HIV+ women might present greater transient acute reductions in BP after resistance exercise.
From a practical point of view, resistance exercises for HIV/AIDS patients are recommended because of the benefits to the musculoskeletal system. Recently, BP responses following a single session of exercise have been considered a predictive tool to identify the chronic cardiovascular effects of training (Tibana et al., 2015). Thus, if similar responses occur in women with HIV, our results suggest that women who have been using HAART for less time, present abdominal obesity, and have a low CD4/CD8 ratio are potentially more likely to present chronic reductions in BP with resistance training.
The present study has some limitations. The lack of a control session (without exercise) is a major limitation and does not allow control for the impact of circadian variation on cardiovascular parameters. It also limits the strength of the identification of the individual responses to exercise, and although we performed several measures during the postexercise period, assumptions should be made with caution (Atkinson and Batterham, 2015). The small sample size limited the comparisons between “responders” and “nonresponders.” Our results are restricted to normotensive women and whether these results are replicable in men and hypertensive patients needs to be investigated. Finally, we investigated the cardiovascular variables for up to 2 hr after an exercise session. Other studies are encouraged to investigate whether changes are maintained under outpatient conditions.
In conclusion, the results of this study indicated a considerable variability in BP responses after a resistance training session. HIV+ women with a higher waist-hip ratio, shorter treatment duration, and lower CD4/CD8 ratio presented higher acute reductions in systolic and diastolic BP.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the Foundation for Research Support of the State of Amazonas (FAPEAM grant #062.03159.2014), National Council for Scientific and Technological Development (CNPQ grant #428555/2016-0) and Araucaria Foundation (grant #40904.425.36484.26072013).