Role of exercise in age-related sarcopenia
Article information
Abstract
Sarcopenia is an age-associated decline of skeletal muscle mass and function and is known to lead to frailty, cachexia, osteoporosis, metabolic syndromes, and death. Notwithstanding the increasing incidence of sarcopenia, the molecular and cellular mechanisms driving age-related sarcopenia are not completely understood. This article reviews current definitions of sarcopenia, its potential mechanisms, and effects of exercise on sarcopenia. The pathogenesis of age-related sarcopenia is multifactorial and includes myostatin, inflammatory cytokines, and mitochondria-derived problems. Especially, age-induced mitochondrial dysfunction triggers the production of reactive oxygen species (ROS) by mitochondria, impedes mitochondrial dynamics, interrupts mitophagy, and leads to mitochondria-mediated apoptosis. Aerobic exercise provides at least a partial solution to sarcopenia as it ameliorates mitochondria-derived problems, and resistance exercise strengthens muscle mass and function. Furthermore, combinations of these exercise types provide the benefits of both. Collectively, this review summarizes potential mechanisms of age-related sarcopenia and emphasizes the use of exercise as a therapeutic strategy, suggesting that combined exercise provides the most beneficial means of combating age-related sarcopenia.
INTRODUCTION
Skeletal muscle is an important organ as it supports the body and enables locomotion. However, skeletal muscle can be degraded by aging, poor nutrition, disuse, and changes in hormonal levels. This phenomenon is called ‘sarcopenia’, which is known to lead to frailty, cachexia, osteoporosis, metabolic syndromes, and death.
Sarcopenia was first presented by Irwin Rosenberg in 1989 to account for age-related muscle mass decline. However, over recent years its definition has been extended to include low muscle mass and poor muscle function. Indeed, the European Working Group on Sarcopenia in Older People proposed that low muscle mass and low muscle function (strength or performance) both be considered essential diagnostic features of sarcopenia (Cruz-Jentoft et al., 2010).
Clinical criteria for sarcopenia vary from country to country, though cutoff points are generally reported in terms of muscle mass, strength, and physical performance. Limb muscle mass is usually estimated by dual energy X-ray absorptiometry and calculated appendicular skeletal muscle mass is then divided by height squared (kg/m2) to produce skeletal muscle mass index (SMI). Sarcopenia is indicated by a SMI >2 standard deviations below the average value for healthy young men or women (Cooper et al., 2013). In addition, SMI has gender-specific cutoff points, that is, 7.26 kg/m2 for men and 5.45 kg/m2 for women (Taaffe et al., 2005). On the other hand, muscle strength is usually assessed by measuring grip strength, which is defined as maximum measured grip strength. The diagnostic criteria for grip strength are 26–30 kg for men and 16–20 kg for women (Heo et al., 2017). Physical performance is usually assessed by measuring habitual gait speed. ‘Sarcopenia with limited mobility’ is described as a habitual gait speed of <1.0 m/sec over a 4-m course or a walking distance of <400 m in a 6-min walk test (Cooper et al., 2013). As mentioned above, the criteria used to define sarcopenia vary from country to country and sarcopenia can be assessed in multiple ways.
POTENTIAL MECHANISMS OF AGE-RELATED SARCOPENIA
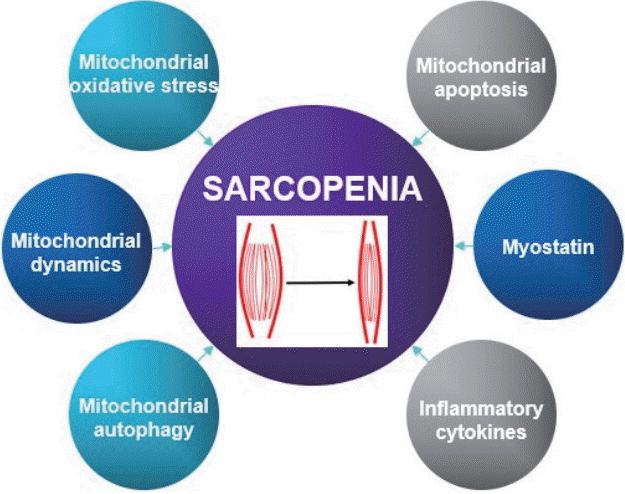
A variety of factors and pathways are involved in the pathogenesis of sarcopenia, such as, environmental causes, endocrine problems, motor neuron loss, activation of inflammatory pathways, and reductions in satellite cell counts (Cruz-Jentoft et al., 2010). Moreover, recent research suggests mitochondrial dysfunction and the activation of apoptotic signaling are critical aspects of the pathogenesis of age-related sarcopenia. In this review, we focus on potential causes of age-related sarcopenia. Fig. 1 summarizes proposed mechanisms.
Mitochondrial reactive oxygen species & mitochondrial dysfunction
Mitochondrial reactive oxygen species (mtROS) is closely related to oxidative stress in aging skeletal muscle and is a major cause of age-induced sarcopenia. The accumulation of mitochondrial ROS in aging skeletal muscle leads to tissue degradation, skeletal muscle atrophy, muscle dysfunction, and increases in fibrous tissue (Heo et al., 2017). mtROS production is associated with mitochondrial DNA (mtDNA) mutations induced by oxidative stress and these mutations result in defective electron transport chain (ETC) components (Alexeyev, 2009). The incorporations of defective subunits into the ETC disrupts oxidative phosphorylation, reduces ATP synthesis, and further increases ROS production (Alexeyev, 2009). Indeed, Wanagat et al. (2001) reported muscle fibers with mtDNA deletions displayed electron transport system abnormalities and fiber atrophy, and Hiona et al. (2010) showed rates of mitochondrial respiration and ATP production were dramatically lower in the skeletal muscles of mtDNA mutant mice. Consequently, age-induced mtROS, mtDNA mutation, and mitochondrial dysfunction are considered potential causes of sarcopenia (Alexeyev, 2009).
Mitochondrial apoptosis
Apoptosis is a highly programmed form of cell death (Leeuwenburgh, 2003) that can be characterized by cell fragmentation, loss of muscle fibers, and muscle atrophy in skeletal muscle. Mitochondria play a major role during apoptosis, and mitochondrial dysfunctions and mtROS trigger the initial events of mitochondria-mediated apoptosis by causing the release of proapoptotic proteins into cytosol (Leeuwenburgh, 2003). Imbalance between pro-apoptotic protein (Bax) and antiapoptotic protein (Bcl-2) in mitochondria induces mitochondrial permeability transition pore (mPTP) opening and the release of cytochrome c from mitochondria to cytosol, which then binds to apoptotic protease-activating factor-1 (Apaf-1) and pro-caspase 9, activates caspase-3, and eventually causes DNA fragmentation (Heo et al., 2017; Leeuwenburgh, 2003). In addition, apoptosis is also triggered by a caspase-independent pathway whereby endonuclease G and apoptosis-inducing factor (AIF) directly trigger DNA fragmentation in mitochondria (Marzetti and Leeuwenburgh, 2006). Previous studies have presented evidence that mitochondrial apoptosis is induced in senescent skeletal muscle. Song et al. (2006) reported the expression of Bax protein is elevated and the expression of Bcl-2 is diminished in senescent skeletal muscle (Song et al., 2006), and similarly Gouspillou et al. (2014) found mPTP was more sensitive in vastus lateralis muscles of older men (Gouspillou et al., 2014). Moreover, Siu et al. (2005) showed dramatic increases in AIF contents and apoptotic DNA fragmentation in gastrocnemius muscles of aged rodents. Thus, mitochondria-mediated apoptosis appears to be a major cause of age-induced sarcopenia.
Mitochondrial dynamics
Function and structure of skeletal muscle fibers are mainly affected by mitochondrial dynamics and morphology (shape and size), which are both induced by intracellular and extracellular signals (Seo et al., 2010a). These changes in the mitochondrial dynamics and morphologies are controlled by continuous fusion and fission. Mitochondrial fusion can compensate for mitochondrial impairment, whereas mitochondrial fission can preserve function by separating dysfunctional mitochondria from healthy mitochondria (Ni et al., 2015). Furthermore, impaired mitochondria may fail their fusion process by inactivating fusion or activating fission machineries and thus prevent damaged mitochondria from being reincorporated into the healthy mitochondrial network (Ni et al., 2015). Thus, mitochondrial dynamics not only determines the shapes of intracellular organelles but also has substantial effects on mtDNA regulation and mitochondrial function. Dynamin-related guanosine triphosphatases, optic atrophy 1 (OPA 1), and mitofusin 1 (Mfn 1) and its paralog mitofusin 2 (Mfn 2) (Seo et al., 2010a) have been shown to be involved in mitochondrial fusion. Mfn 1 and Mfn 2 in the outer mitochondrial membrane tether adjacent mitochondria, whereas OPA 1 in the inner mitochondrial membrane mediates inner mitochondrial membrane fusion (Archer, 2013). Westermann identified the proteins involved in mitochondrial fission as dynamin-related protein 1 (Drp 1) and fission protein (Fis 1) (Westermann, 2010). Imbalances of mitochondrial dynamics negatively affect mitochondrial homeostasis and function, and it has been recently reported that in skeletal muscle these imbalances induce senescence and muscle atrophy. For example, Chen et al. (2010) reported that deletion of Mfn 1 and Mfn 2 led to mtDNA mutation, and that accumulations of mtDNA mutations resulted in mitochondrial dysfunction and muscle atrophy. In addition, Romanello et al. (2010) observed overexpression of Drp 1 and Fis 1 triggered mitochondrial fragmentation and dysfunction, activated mitochondrial autophagy (mitophagy), and caused muscle fiber atrophy.
Mitochondrial autophagy
Mitophagy is type of autophagy that results in the removal of unnecessary or impaired mitochondria. Mitophagy usually begins when membrane potential in skeletal muscle is lost because of aging and is preceded by mitochondrial fission. Recently, mitophagy in skeletal muscle has received greater research attention, especially in the context of muscle atrophy (Yan et al., 2012). Several authors have suggested that mitophagy dysfunction may not be properly utilized due to aging, considering observations of reduced mitochondrial biogenesis and continuous accumulations of damaged organelles (Heo et al., 2017). For example, it has been reported the expressions of autophagy related genes, such as, LC3, Atg7, p62, Beclin 1, Bnip 3, Parkin are reduced by aging (Heo et al., 2017). In addition, Romanello et al. (2010) reported BNIP3 overexpression induced mitochondrial fragmentation, higher levels of autophagy, and muscle atrophy (Joseph et al., 2013; Romanello et al., 2010), and Pagano et al. (2015) reported higher expressions of Beclin 1 and LC3 II in skeletal muscles of sarcopenic 15- to 22-years-old dogs than 2- to 5-years-old dogs. Collectively, it would appear mitophagy is critical for the maintenance of mitochondrial function and muscle mass.
Myostatin
Myostatin is an extracellular cytokine and a member of the transforming growth factor β superfamily, playing a negative role in regulating skeletal muscle mass and growth (Elkina et al., 2011). During embryogenesis, myostatin is exclusively expressed in skeletal muscle and controls the differentiation and proliferation of myoblasts (Elkina et al., 2011) by inhibiting the expression of insulin-like growth factor (IGF-1) or of follistatin, which is known to be positively related with muscle hypertrophy. Furthermore, it has been reported myostatin is associated with aging. Indeed, Yarasheski et al. (2002) reported that increases in serum myostatin levels were highest in physically frail older women and that they were inversely associated with skeletal muscle mass (White and LeBrasseur, 2014). Siriett et al. (2007) showed that myoD and Pax7 (potent markers of myogenesis) protein levels were significantly elevated in gastrocnemius muscles from aged mice treated with a myostatin antagonist. However, several authors have failed to demonstrate age-related changes in myostatin mRNA levels in skeletal muscle or in circulating myostatin-immunoreactive protein levels (White and LeBrasseur, 2014). Thus, it seems further studies are needed to resolve conflicting results regarding the relation between myostatin and aging.
Inflammatory cytokines
It has been demonstrated inflammatory markers contribute to age-related muscle wasting (Budui et al., 2015). For example, elevated levels of tumor necrosis factor alpha (TNF-α) were found to increase muscle catabolism by suppressing the Akt/mammalian target of rapamycin (mTOR) pathway (Budui et al., 2015). It also seems inflammatory cytokines may antagonize the anabolic effect of IGF-1 by inducing the development of growth hormone resistance, which decreases both circulating and muscle IGF-1 levels (Budui et al., 2015). However, the effects of these cytokines may be more complex because interleukin 6 (IL-6) may play a role, and it can act as pro- or anti-inflammatory cytokine (Rolland et al., 2008). Recent experimental studies have suggested that IL-6 in blood can be differentiated from muscle-derived IL-6, which can inhibit TNF-α (Rolland et al., 2008). The involvements of cytokines in sarcopenia remain to be clarified, but nonetheless, sarcopenia appears to be a cytokine-associated aging phenomenon (Rolland et al., 2008).
EFFECTS OF EXERCISE ON SARCOPENIA
Exercise is essential for health because it increases muscle mass, reduces body fat, and improves muscle strength, endurance, immune function, and the cardiovascular system. Accordingly, exercise should be considered an essential feature of therapeutic strategies targeting age-related sarcopenia. Here, we briefly describe the effects of aerobic, resistance, and combined exercises on age-related sarcopenia.
Aerobic exercise and sarcopenia
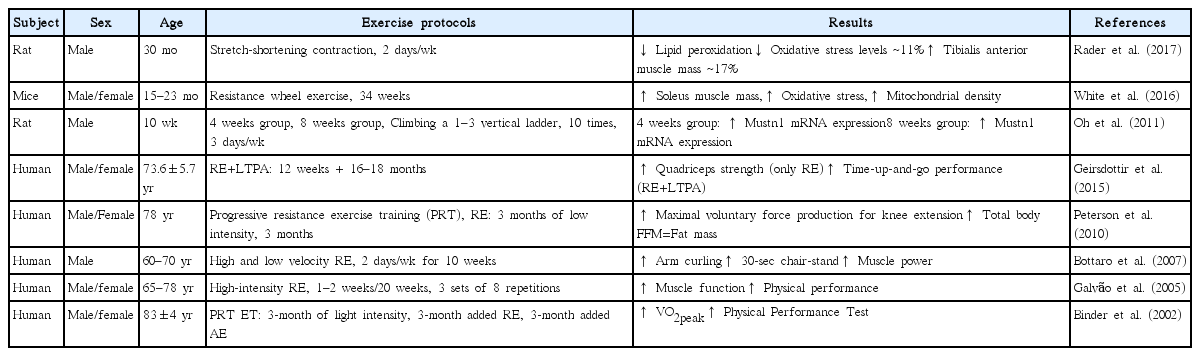
Aerobic exercise causes ATP production in mitochondria within skeletal muscle, and improves aerobic capacity, metabolic regulation, and cardiovascular function. Furthermore, it contributes to the inductions of mitochondrial biogenesis and dynamics, to the restoration of mitochondrial metabolism, reduces the expressions of catabolic genes and increases muscle protein synthesis (Erlich et al., 2016; Konopka and Harber, 2014; Seo et al., 2016). Previous studies have shown endurance exercise training may suppress the apoptotic pathway in skeletal muscle and that aerobic exercise helps maintain the expression of autophagy protein and may even increase the expressions of autophagy-related proteins in skeletal muscle (Yan et al., 2012). In addition, several authors have shown aerobic exercise controls mRNA expression of myostatin (Ko et al., 2014). Given that these molecular factors are associated with age-related sarcopenia, it seems aerobic exercise has a protective effect. Indeed, Harber et al. (2012) reported that cycle exercise increased muscle size and strength in both 20-years-old and 74-years-old subjects. Moreover, Bori et al. (2012) reported that 12 weeks of aerobic exercise training enhanced mitochondrial biogenesis and mitochondrial fission protein (Fis 1) of older subjects. Collectively, aerobic exercise appears to ameliorate mitochondria-related problems and improve muscle hypertrophy and strength. Table 1 summarizes the effects of aerobic exercise on age-related sarcopenia.
Resistance exercise and sarcopenia
Resistance exercise is considered an important strategy for preventing muscle wasting because it stimulates muscle hypertrophy and increases muscle strength (Johnston et al., 2008) by shifting the balance between muscle protein synthesis and degradation towards synthesis (Johnston et al., 2008). It is known regular resistance exercise increases the sizes and cross-sectional areas of muscle fibers, especially fast-twitch fibers (types IIa and IIx) rather than slow-twitch fibers (type I) (Heo et al., 2017). Increases in muscle protein synthesis and muscle fibers hypertrophy increase force-generating ability (Johnston et al., 2008), muscle quality, and physical performance. However, resistance exercise has several limitations. In particular, it has a little effect on the expressions of mitochondrial proteins or their functions, and these are considered potential causes of age-related sarcopenia. Nonetheless, resistance exercise is a meaningful exercise prescription for sarcopenia in terms of improving muscle mass and function. Binder et al. (2002) showed that progressive resistance training resulted in increased physical performance and peak oxygen uptake. Bottaro et al. (2007) reported that 10 weeks of resistance exercise enhanced physical activities, including arm curling and 30-sec chair-stand. In addition, Peterson et al. (2010) showed 3 months of resistance exercise improved maximal force production of knee extension and total body fat free mass. Table 2 provides a summary of the effects of resistance exercise on age-related sarcopenia.
Combined exercise and sarcopenia
The majority of studies on the effects of exercise have focused on either aerobic or resistance exercise. As mentioned above, aerobic exercise has a little effect on muscle strength or mass compared with resistance exercise (Lee, 2017; Takeshima et al., 2004), whereas resistance exercise can increase the risk of injury, reduce participation rates, and induce boredom because of the extent of repetition (Lee, 2017). Also, resistance exercise can be less effective in older individuals because of deficient mTOR signaling, which is involved in muscle protein synthesis (Heo et al., 2017). Accordingly, no single type of exercise would seem to address adequately the requirements of therapeutic exercise in age-related sarcopenia, and thus, it has been recommended well-rounded exercise programs consisting of aerobic and resistance exercises should be preferred (Takeshima et al., 2004). For example, a circuit exercise program has been developed that combines these two exercise types (Lee, 2017; Takeshima et al., 2004). Recently, Lee et al. (2017) reported that 12 weeks of circuit program improved walking and balancing abilities and isokinetic muscle functions. Gudlaugsson et al. (2013) showed ‘multimodal training interventions’ conducted on 117 elderly subjects for 6 months improved endurance performance as determined by 6-min walking test. Collectively, these reports indicate regular combined exercise can be utilized to combat age-related sarcopenia. Further research is needed to determine whether combined exercise retards potential molecular mechanisms of age-related sarcopenia. Table 3 presents a summary of the effects of combined exercise on age-related sarcopenia.
CONCLUSIONS
Mitochondrial oxidative stress, apoptosis, and dynamics, and mitophagy, myostatin, and inflammatory cytokines are all believed to be associated with age-related sarcopenia. Nevertheless, aerobic, resistance, and combined exercise training regimes have been shown to produce the most beneficial preventive and therapeutic effects. Further research is required to elucidate the cellular and molecular mechanisms responsible for protective effect of regular exercise training on age-induced sarcopenia of skeletal muscles.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by Inha University Research Grant (2017).