Simulated altitude exercise training damages small intestinal mucosa barrier in the rats
Article information
Abstract
This study investigated the effect of simulated altitude training on the changes of small intestinal mucosa barrier, bacterial overgrowth and inflammatory response in the small intestine of rat. Male 8-week-old Sprague-Dawley rats were randomly divided into four groups: normal oxygen sedentary group (n=30), normal oxygen exercise group (n=30), low oxygen sedentary group (n=30) and low oxygen exercise group (n=30). Exercise training was on a treadmill for 1 hr per day on days 3, 6, and 9 in the hypoxia condition. Hematological profiles, hematolxylin and eosin staining, fluorescence in situ hybridization, reverse transcription-polymerase chain reaction and Western blot were used to analyze the effect of simulated altitude training on the amount of bacteria, and expression of mRNA and protein. Simulated exercise training significantly increased red blood cells and hematocrit. The small intestinal mucosa barrier was significantly injured by the simulated altitude exercise training. Comparatively more bacterial growth was evident in the small intestine by the simulated altitude exercise training. mRNA levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) and protein expression of nuclear factor-kappa B (NF-κB) were significantly elevated by simulated altitude exercise training. These results suggest that the simulated altitude exercise training may impair the small intestinal mucosa barrier via elevation of bacterial growth and inflammatory cytokines (IL-6, TNF-α) and the up-regulation of NF-κB in the rats.
INTRODUCTION
High altitude is a special geographic environment that incorporates low pressure and low oxygen levels. The hypoxic environment has been exploited in athletic training in an effort to improve performance (Ashenden et al., 1999; Saunders et al., 2009; Wilber, 2001). The positive effect of altitude training on athletic performance includes increased rate of oxygen utilization, due to increased hemoglobin content and increased oxygen carrying capacity (Pottgiesser et al., 2009; Wachsmuth et al., 2013).
Darkening this picture, several studies reported the association of altitude training with loss of appetite, vomiting, diarrhea, and other gastrointestinal symptoms (Flaherty et al., 2016; Tschöp et al., 1998). Another consequence of prolonged endurance or intense exercise training could be a decrease in the intestinal capacity to avoid infection. Hypoxia conditions may injure the small intestinal antioxidant system and could alter gastrointestinal mucosa microstructure (Deitch, 2002; Maeda et al., 2010; Sun et al., 2004).
Under normal circumstances, the intestinal microecological structure maintains relative balance and stability. With the help of the intestinal mucosa barrier and the immune system, the resident intestinal flora avidly adheres to the intestinal mucosa, resulting in colonization resistance (Garrett et al., 2010). Pathological changes, such as altitude exposure, result in intestinal problems that include derangement of the symbiosis between the host and the normal flora, and imbalance of intestinal microecologies; these changes lead to intestinal mucosa barrier damage (Round and Mazmanian, 2009). The gastrointestinal tract appears ischemic and hypoxic in the earliest stages of stress (Gosain and Gamelli, 2005). Hypoxic ischemia leads to intestinal mucosa epithelial edema, disrupted mucosal thickness and increased intestinal permeability, which induce endotoxin and bacterial translocation (Nagpal et al., 2006; Yu et al., 2013). Bacteria and endotoxin enter the portal vein or lymphatic system and translocate to other tissues and organs, such as liver, leading to a cascade response mediated by inflammatory mediators, including tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6), which are downstream factors of nuclear factor-kappa B (NF- kB) (Fan et al., 2015). They play an important role in a variety of inflammatory reactions and are correlated with the severity of inflammation (Ferencz et al., 2006). High altitude hypoxia and intense exercise training independently trigger injury of intestinal mucosa. Therefore, altitude exercise training may cause more severe of intestinal mucosa damage because of the dual simulation of hypoxia and exercise training.
Previous studies have been shown that the altitude hypoxia can damage of intestinal epithelium and increase the levels of inflammatory cytokines. However, the link between altitude training in intestinal mucosa barrier damage and inflammatory responses is unclear. The purpose of this study is to investigate the effect of altitude training on small intestinal barrier damage and levels of TNF-α, IL-6, and NF-kB in rats.
MATERIALS AND METHODS
Animals
All experiments were performed on 8-week-old male Sprague-Dawley rats (Vital River Laboratory Animal Technology Co., Ltd, Beijing, China). Rats were housed under controlled temperature (22°C–25°C), humidity (75%) and light conditions (alternating 12-hr light-dark cycle) and were provided food and water ad libitum. The rats were randomly assigned to four groups: normal oxygen sedentary group (NOS, n=30), normal oxygen exercise group (NOE, n=30), low oxygen sedentary group (LOS, n=30), and low oxygen exercise group (LOE, n=30). The low oxygen groups were housed in a hypoxic chamber with 12.7% oxygen to simulate the hypoxic environment at an altitude of 4,000 m. All protocols were approved by the institutional animal care and use committee of Beijing Sport University (2015ZD006).
Exercise training protocol
A motorized rodent treadmill was used for exercise training. Rats were allowed to adapt to the exercise training under the normal oxygen condition with moderate exercise intensity (60%–70% maximal oxygen uptake) for 5 days. After that, animals were randomly assigned into normal oxygen exercise and low oxygen exercise group. According to the Bedford protocol (Bedford et al., 1979), animals were trained at 24 m/min, 45 min/day in moderate intensity on days 3, 6, and 9 until the experimental endpoint.
Tissue isolation
Rats were sacrificed after the formal exercise training on days 3, 6, or 9. The small intestine was isolated under anesthesia achieved by an intraperitoneal abdominal injection of 3% pentobarbital (30 mg/kg). Blood was collected from the abdominal artery in the presence of an anticoagulation compound and biochemical tests were done within 6 hr.
Hematoxylin and eosin staining
Hematoxylin and eosin staining (H&E) staining was used to measure small intestine epithelium. Tissues were fixed in 4% formaldehyde for 24 hr and embedded in paraffin. Serial cross-sections (5-μm thickness) were cut using a model RM 2235 microtome (LEICA, Nussloch, Germany) and were examined under light microscopy using an Eclipse Ni-E microscope (Nikon, Tokyo, Japan). The height and the surface area of small villous and the thickness of mucosa were measured by ImageJ software (National Institute Health, Bethesda, MD, USA). Eight numbers of villous were measured in each H&E section.
Florescence in situ hybridization
EUB-338 probe (5′-GCTGCCTCCGTAGGAGT-3′, 1 μg/mL) bound to fluorescein isothiocynate (FITC) (BoGoo Biotechnology Co., Ltd., Shanghai, China) was used to detect the bacteria in the small intestine. The FITC has excitation and emission wavelengths of 494 and 518 nm, respectively, and emits green fluorescence. The probe binds to a conserved region of bacterial 16S rRNA that is specific for Eubacteria. Tissue sections were counterstained with 5 mg/mL 4′,6-diamidino-2-phenylindole (DAPI; Biotime, Beijing, China) to visualize eukaryotic cell nuclei. Imaging was performed using a LSM710 confocal microscope (Carl Zeiss, Jena, Germany) at ×200 magnification with image data analyzed using Image pro Plus image software.
RT-PCT
Total RNA was isolated from intestine using RNA isolation Kit (Tiangen Biotech Co., Ltd., Beijing, China). Reverse transcription of mRNA was achieved using an ImProm-II Reverse Transcription System (Promega, Madison, WI, USA). The levels of beta-actin RNA were used as endogenous controls for normalization rat RNA. The relative gene expressions were calculated using cycle threshold (Ct) values in accordance with the ΔCt method. Primer sequences were as follows: Beta-actin, forward: 5′-CCCATCTATGAGGGTTACGC-3′, backward: 5′ TTTAATGTCACGCACGATTTC-3′; TNF-α, forward: 5′-GAGATGTGGAACTGGCAGAGGA-3′; backward: 5′-TCAGTAGACAGAAGAGCGTGGTG-3′; and IL-6, forward: 5′-CCTACCCCAACTTCCAATGCT-3′, backward: 5′-GGTCTTGGTCCTTAGCCACT-3′.
Western blot
Small intestine tissues were homogenized in lysis buffer (50 mM Tris and pH 7.4, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 2 mM sodium pyrophosphate, 25 mM β-glycerophosphate, 1 mM ethylenediaminetetraacetic acid, 1 mM Na3VO4, 0.5-μg/mL leupeptin; Solarbio Science & Technology Co., Ltd. Beijing, China) and centrifuged for 5 min at 12,000 rpm and 4°C. The supernatant was removed and protein in the pellet was quantified using a BCA assay Kit (Solarbio Science & Technology Co., Ltd.). Proteins (30 μg) were resolved using 12% SDS-polyacrylamide gel electrophoresis and transferred to a polyvinylidene fluoride membrane. Nonspecific reaction of the membrane was removed by blocking it for 1 hr on ice in 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS). NF-κB (Cell Signaling Technology, Waltham, MA, USA) and β-actin (Cell Signaling Technology) were incubated overnight at 4°C in TBS with 3% BSA. Horseradish peroxidase-labeled secondary antibody (Biotime) was diluted 1:5,000 and added. Blots were developed for visualization using an enhanced chemiluminescence detection kit (Thermo Fisher, San Diego, CA, USA). GIS software (Tanon, Shanghai, China) was used to quantify expression.
Statistical analyses
Values are expressed as mean±standard error of the mean. The statistical evaluation was conducted with a one-way analysis of variance and two-way repeated measured analysis of variance of variance followed by a Duncan’s post hoc test, and P<0.05 was considered statistically significant.
RESULTS
Effect of simulated altitude training on body weight
In order to investigate the effect of simulated altitude exercise training on body weight, we’ve analyzed the changes of body weight in normal and low oxygen conditions (Fig. 1). Significantly restrained persistent increasing of body weight under the normal oxygen and low oxygen conditions compared with sedentary groups (P<0.05, NOS vs. NOE; LOS vs. LOE, Fig. 1). There was significantly greater inhibition of body weight increase in the simulated exercise training group compared with the normal oxygen exercise group.
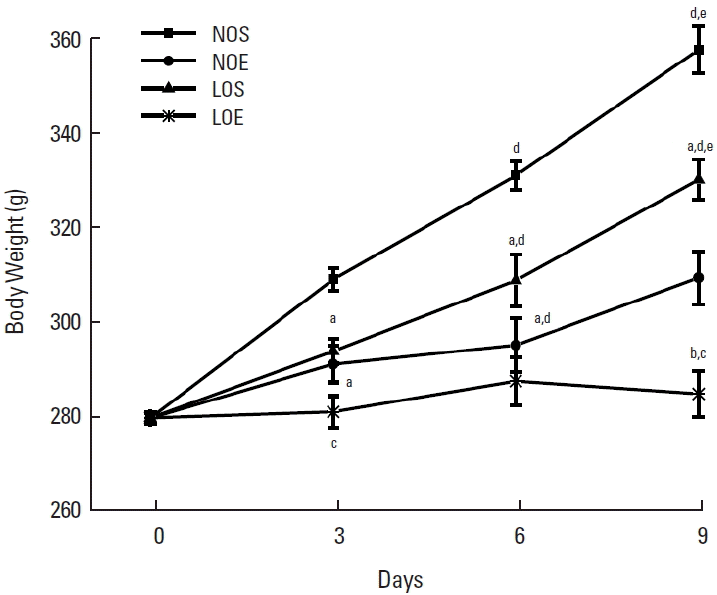
Simulated altitude training inhibited the elevation of body weight in rats, both under the normal oxygen and low oxygen conditions. NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. Each data indicated mean±standard error of the mean (n=10). aP<0.05 vs. NOS. bP<0.05 vs. NOE. cP<0.05 vs. LOS. dP<0.05 vs. day 3. eP<0.05 vs. day 6.
Effect of simulated altitude training on hematological profiles
In whole blood, red blood cells (RBCs) and the percentage of hematocrit (HCT) were significantly enhanced in LOS and LOE groups compared with NOS and NOE groups (P<0.05) (Fig. 2). Also the low oxygen exercise training group significantly increased RBC number but decreased the percentage of HCT compared with low oxygen sedentary at day 6, respectively (P<0.05, LOS vs. LOE). Considering training time as an influential factor, RBC and HCT were compared at different times. No temporal relationship was apparent for RBC but was suggested for HCT. The NOE and NOS groups were similar.
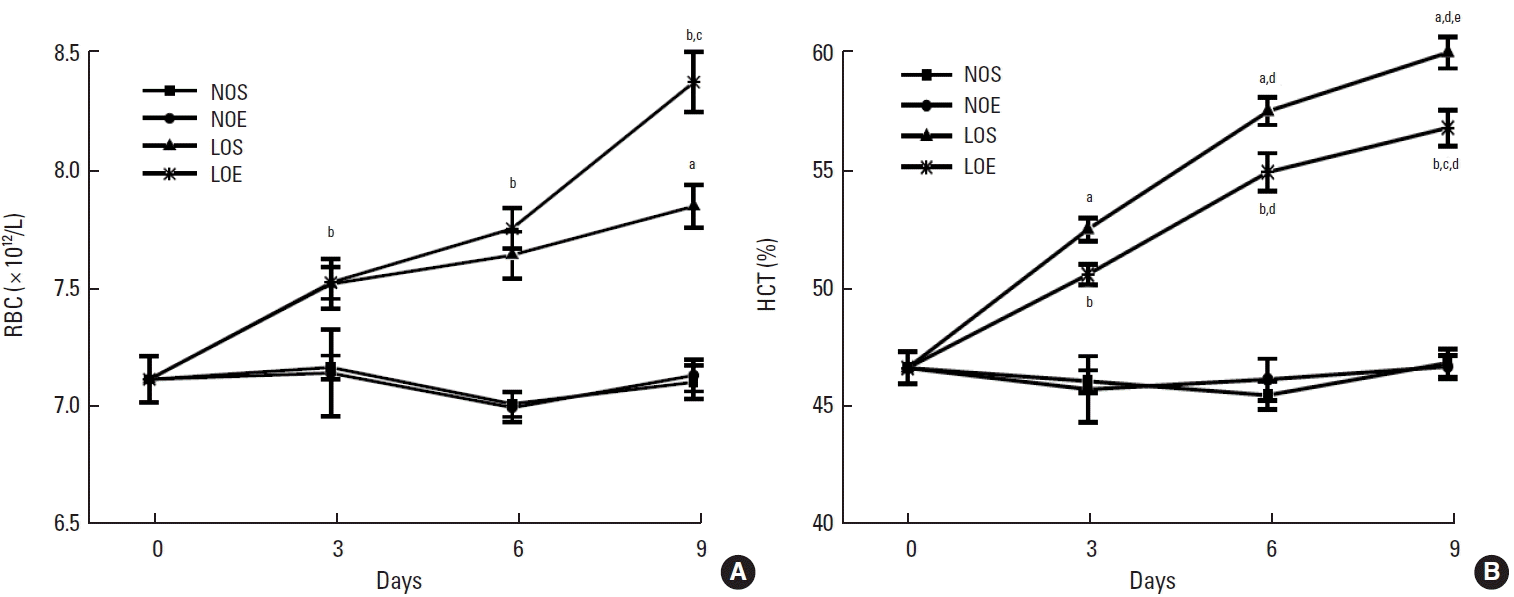
Simulated altitude training enhanced the elevation of haematological parameters: (A) red blood cells (RBCs), (B) hematocrit (HCT). NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. Each data indicated mean±standard error of the mean (n=10). aP<0.05 vs. NOS. bP<0.05 vs. NOE. cP<0.05 vs. LOS. dP<0.05 vs. day 3. eP<0.05 vs. day 6.
Effect of simulated altitude training on intestinal mucosa barrier
We compared the small intestine in the NOS, NOE, LOS, and LOE groups at different times (Fig. 3). Microscopic examinations revealed pathological changes in the villi. Obviously swelling of villi were evident in tissue obtained from the LOE group at days 3, 6, and 9 (P<0.05) (Fig. 3). H&E staining additionally revealed peeling of tissue from the submucosa. Congestion was evident in different locations including submucosa and the periphery of villi. The most severe damage of the intestinal mucosal barrier was evident at day 6 in the LOE group.
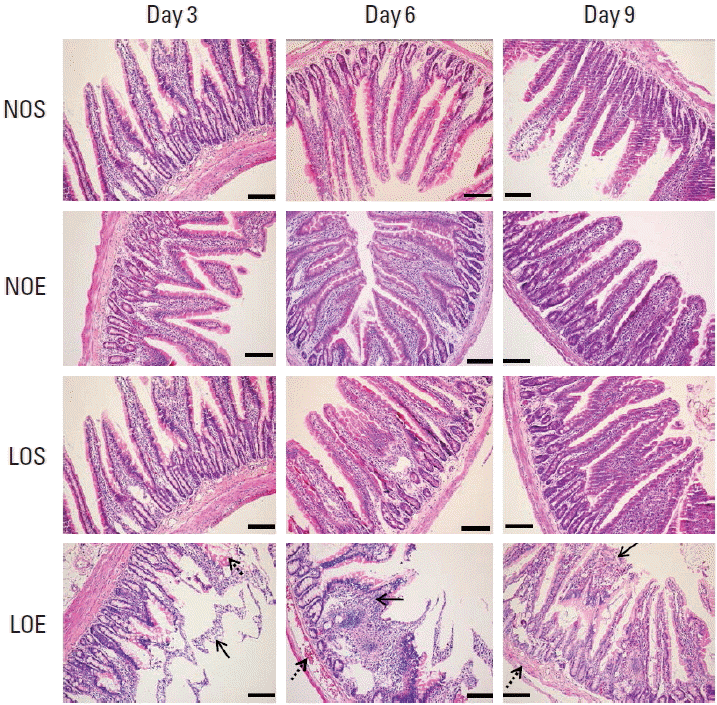
Simulated altitude training damaged small intestinal mucosa barrier. NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. Solid arrows indicate villi swelling and peeling from submucosa; dotted lines indicate congestion. Scale bar is 100 μm.
Effect of simulated altitude training on bacteria in the small intestine
Florescence in situ hybridization analysis shows that EUB-338 positive bacteria were significantly increased in LOS and LOE groups compared with NOS and NOE groups (P<0.05) (Fig. 4) and EUB-338 positive bacteria of LOE group was significantly increased compared with LOS group at the same times (P<0.05) (Fig. 4).

Simulated altitude training increased the amount of bacteria in small intestine. (A) Fluorescence in situ hybridization presented and located bacteria, (B) mean density of 5′-GCTGCCTCCGTAGGAGT-3′ (EUB-338) probe positive fluorescence. Each data indicate mean±standard error of the mean (n=10). NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. DAPI (4′,6-diamidino-2-phenylindole), blue; EUB-338 probe, green. aP<0.05 vs. NOS. bP<0.05 vs. NOE. cP<0.05 vs. LOS.
Effect of simulated altitude training on mRNA levels of TNF-α and IL-6
IL-6 and TNF-α mRNA levels of small intestine in the LOE group were significantly increased compared with the NOE and LOS groups, respectively (P<0.05) (Fig. 5). Significant time-dependent changes of the levels of IL-6 and TNF-α levels were evident in the LOS and LOE groups.

Simulated altitude training increased mRNA levels of interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) in small intestine. (A) Change of mRNA levels of IL-6, (B) change of mRNA levels of TNF-α. Each data indicate mean±standard error of the mean (n=10). NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. aP<0.05 vs. NOS. bP<0.05 vs. NOE. cP<0.05 vs. LOS. dP<0.05 vs. day 3. eP<0.05 vs. day 6.
Effect of simulated altitude training on small intestine NF-κB
NK-κB expression was significantly increased in the LOS and LOE group compared with the NOS and NOE groups at the same times, respectively (P<0.05) (Fig. 6). Significantly increasing NF-κB was evident in the LOE group on day 6, but statistical significance was not evident at days 3 and 9.
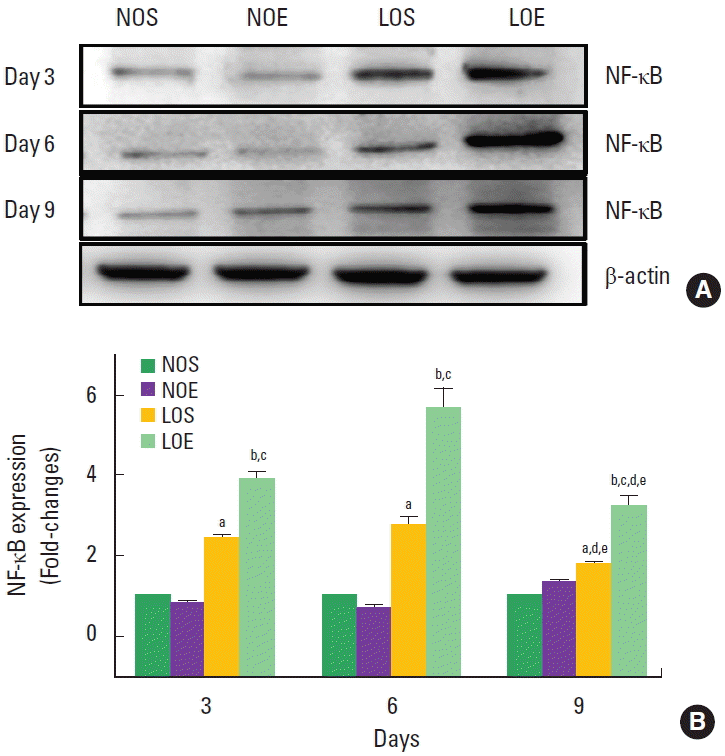
Simulated altitude training increased protein expression of nuclear factor-kappa B (NF-κB) expression in small intestine. (A) Western blot showed NF-κB. (B) Fold-changes of NF-κB for each group. Each data indicate mean±standard error of the mean (n=10). NOS, normal oxygen with sedentary; NOE, normal oxygen with exercise training; LOS, low oxygen with sedentary; LOE, low oxygen with exercise training. aP<0.05 vs. NOS. bP<0.05 vs. NOE. cP<0.05 vs. LOS. dP<0.05 vs. day 3. eP<0.05 vs. day 6.
DISCUSSION
There are three major findings. Simulated altitude training damaged the small intestinal mucosa barrier, increased the amount of bacteria in small intestine and elevated the levels of IL-6 and TNF-α due to the up-regulation of NF-κB in the small intestine.
In general, people tend to display symptoms of hypoxia at altitudes exceeding 2,000 m, with symptoms being more severe in those who experience a greater change in altitude, such as from sea level. RBC and HCT are used to evaluate the effect of altitude training adaptation in athletes or animal models. The variations of these blood factors are involved in hypoxia and altitude training. Most often, hypoxia or altitude conditions will result in increased RBC and HCT (Bor-Kucukatay et al., 2014 ; Stokke et al., 1986). The present results from simulated altitude training reaffirm the view that a low oxygen environment boosts RBC and HCT.
In the early days after exposure to high altitude, about half of all people will experience nausea, vomiting, diarrhea and other gastrointestinal symptoms, collectively termed “high altitude gastrointestinal stress syndrome” (Anand et al., 2006). This syndrome is closely related with hypoxia-induced intestinal mucosa barrier dysfunction. Hypoxic exposure is a physiological stimulus; its biological impact on the body mainly depends on the length and intensity of hypoxia exposure. Acute or persistent hypoxic exposure causes intestinal mucosa injury more easily (Kannan et al., 2011). Recavarren-Arce et al. (2005) examined gastric biopsy samples and described more serious gastrointestinal mucosa injuries in Peruvians dwelling in the Andes at an altitude of 3,700 m compared with individuals living at sea level through gastric biopsy. Zhang et al. (2015) indicated the link between morphological changes of the small intestine after rapid entry to high altitudes; villi swelling and hyperemia of small intestinal lamina propia with visible inflammatory granulocytes were evident.
The normal intestinal barrier include a mechanical barrier of intestinal mucosa epithelium, immune barrier, chemical barrier and biological barrier. Our research mainly focused on the effect of simulated altitude training on the mechanical intestinal mucosa barrier. H&E staining revealed swelled small intestinal villi and shortened villi length in the LOE group on days 6 and 9, respectively. Microscopic observation revealed mild pathological changes at day 9 compared with day 6 in the LOE group. These results suggest hypoxia resistance among the LOE rats depending on the duration of hypoxia exposure.
The intestinal mucosa barrier comprises epithelial cells and tight junction. The latter guards against bacterial invasion into intestinal lamina propria, blocks the gap between adjacent epithelial cells and maintains the stability of the intestinal mucosal barrier function to avoid abnormal mucosal immune response (Groschwitz and Hogan, 2009). When the body encounters stress, the gastrointestinal tract reacts first to ischemia and hypoxia (Secchi et al., 2000). Thus, the gastrointestinal tract is more prone to injury, such as intestinal mucosa atrophy. On the other hand, the intestinal tract immunity organ is more sensitive to physical or environmental stress. Compared with the sea level environment, altitude hypoxia exposure is more excessive. This will diminish intestinal tract function and promote translocation of invading pathogenic bacteria and endotoxin into blood and other organs through the damaged intestinal mucosa barrier (Boros et al., 1995; Pedersen and Hoffman-Goetz, 2000; Rowell, 1983).
The endotoxin and bacteria induce intestinal endotoxemia. Endotoxin translocation is more common than bacterial translocation. Endotoxin translocation leads to a vicious cycle that aggravates intestinal mucosa barrier damage (Wang et al., 2004). Once the intestinal barrier is breached, damage can be caused by invading pathogens or from overgrowth of endogenous pathogens, such as bacteria. Invasive bacteria can damage the mucosal layer and intestinal epithelial cells as well as the lamina propria (Zeitouni et al., 2016). This process can be considered a negative-interactive feedback. We used the EUB338 probe to detect bacteria in the small intestine. The results indicated that hypoxia and altitude training induced an imbalance in the flora of the small intestine. The number of microorganisms was highest at day 3 in the LOS and LOE groups.
High altitude hypoxia injure the intestinal mucosal barrier includes swelling and decreased length of villi in rats (Zhang et al., 2015). These findings provide a cautionary note for athletes engaged in high altitude training. Another study examined the expression levels of Toll-like receptor (TLR)/NF-κB, IL-6, and TNF-α, and the ultrastructure of the small intestinal mucosa in a rat model of hypoxia (Luo et al., 2012). These findings indicate that hypoxia can induce increased expression of NF-κB mRNA and protein. The authors concluded that hypoxia-induced small intestinal barrier damage and bacterial translocation might underlie the TLR/NF-κB signaling pathway. In the present study, we detected the expression of NF-κB protein and mRNA levels of TNF-α and IL-6. All were sharply increased by low oxygen and simulated altitude training. Interestingly, NF-κB expression was suppressed in the NOE group compared with the NOS group.
As a transcription factor, NF-κB stimulates the activity of a variety of inflammatory cytokines and induces the copious release of inflammatory factors. These mediators induce apoptosis of intestinal epithelial cells and injury of intestinal tract tissue, and have been implicated with organ dysfunction. TNF-α and IL-6 are pivotal inflammatory factors located downstream of NF-κB. They play an important role in multiple inflammatory reactions and are closely related to the severity of inflammation (Spehlmann and Eckmann, 2009).
IL-6 is another inflammatory mediator and induced by NF-κB activation. Also, NF-κB is critical for TNF-α-induced inflammatory reactions. The function of the intestinal barrier may be regulated by inflammatory cytokines such as TNF-α and IL-6 (Topol and Kamyshny, 2013). In particular, they can accelerate the inflammatory reaction of intestinal organs under hypoxic state that mediate the inflammatory response and damage the function of the intestinal barrier.
Altitude training is widely used by professional athletes to improve performance. The present results add to the concern of the potential damage of this pursuit on inflammatory damage of the gastrointestinal tract. The specific signaling pathway of NF-κB and the possibility of bacterial or endotoxin translocation through the breached intestinal mucosal barrier should be investigated.
Taken together, these data suggest that the simulated altitude exercise training may impair the small intestinal mucosa barrier, which allows increasing of growth of bacteria by elevation of IL-6, TNF-α, and NF-κB in intestine of rats.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Education of the Republic of Korea and the National Research Foundation of Korea (NRF-2017S1A5A2A01026677).
