Paternal physical exercise improves spatial learning ability by enhancing hippocampal neuroplasticity in male pups born from obese maternal rats
Article information
Abstract
Maternal obesity exerts negative effects on cognitive function and behavior of the offspring. In the present study, we assessed the effects of paternal physical exercise on spatial learning ability in relation with hippocampal neuroplasticity in the rat pups born from the obese maternal rats. There were four experimental groups: paternal nonexercised male pups from normal maternal rats, paternal exercised male pups from normal maternal rats, paternal nonexercised male pups from obese maternal rats, and paternal exercised male pups from obese maternal rats. Normal diet was supplied for normal maternal rats and high-fat diet was supplied for obese maternal rats for a 12-week period until mating, and the same diet for each group continued throughout pregnancy and lactation period. Male rats in the exercising groups exercised for a 12-week period. Spatial learning ability was reduced in the male rat pups born from the obese maternal rats. Expressions of brain-derived neurotrophic factor (BDNF) and tyrosine kinase B receptor (TrkB) in the hippocampus were suppressed and cell proliferation and differentiation in the hippocampus were reduced in the male rat pups born from the obese maternal rats. Paternal treadmill exercise improved spatial learning ability, increased BDNF and TrkB expressions, and enhanced cell proliferation and differentiation in the male rat pups born from the obese maternal rats. It can be suggested that paternal exercise enhances hippocampal neuroplasticity and consequently improved spatial learning ability in the rat pups born from the obese maternal rats.
INTRODUCTION
Obesity is a risk factor not only for the cardiovascular diseases but also for the brain-related disorders (Elias et al., 2005; Wolf et al., 2007). Nutritional supply in the early stage after birth depends on the maternal conditions and excessive consumption of certain nutrients by mothers exerts detrimental effect on the brain functions of offspring (Férézou-Viala et al., 2007; Tozuka et al., 2009a; Tozuka et al., 2010). Nutrients, hormones, and growth factors are transported from the mother to the offspring through the placenta or breastfeeding (Kodomari et al., 2009; Raiten et al., 2007). Maternal obesity exerts negative effects on cognitive function and behavior of offspring (Tozuka et al., 2009a, 2009b).
Hippocampus is a key part of the brain involved in the processes of learning process and memory formation. Active neurogenesis occurs in the hippocampus after birth and hippocampus is the brain area with greatest neuroplasticity (Fuchs and Gould, 2000; Lie et al., 2004).
Brain-derived neurotrophic factor (BDNF) has emerged as a key regulator of activity-dependent synaptic plasticity, and BDNF plays a key role in spatial learning ability (Donovan et al., 2008). BDNF controls development, survival, and differentiation of the neurons through the tyrosine kinase B receptor (TrkB) (Bramham and Messaoudi, 2005). 5-Bromo-2′-deoxyuridine (BrdU) is a synthetic nucleoside that is an analog of thymidine. BrdU is commonly used in the detection of proliferating cells in living tissues (Lehner et al., 2011). Doublecortin (DCX) is a marker for the cellular differentiation and DCX is associated with structural plasticity in the adult mammalian brain (Kim et al., 2013a).
Maternal exercise during pregnancy enhanced brain development of offspring by increasing neurogenesis through enhancing BDNF expression (Kim et al., 2007). Maternal exercise during pregnancy improved learning and memory in rat pups (Akhavan et al., 2008). There are many on-going studies on the relationship between maternal exercise and cognitive function of offspring.
The maternal effect is considered as an important form of the parental influence, in contrast, the paternal effect is often relatively overlooked. Half of the paternal genome is passed down to the next generation (Krawetz, 2005), therefore, the paternal conditions also should be considered as the important factor for the development of offspring. In the present study, we assessed the effects of paternal physical exercise on spatial learning ability in relation with hippocampal neuroplasticity in the rat pups born from the obese maternal rats.
MATERIALS AND METHODS
Animals and treatments
All animal experimental procedures conformed to the regulations stipulated by the National Institutes of Health and the guidelines of the Korean Academy of Medical Science. This study was approved by the Kyung Hee University Institutional Animal Care and Use Committee (KHUASP [SE]-14-018).
Male and female Sprague-Dawley rats were used (4 weeks old) for this experiment. Normal diet was supplied for normal maternal rats and high-fat diet (60%) was supplied for obese maternal rats for a 12-week period until mating, and the same diet for each group continued throughout pregnancy and lactation period. Male rats in the exercising groups exercised for a 12-week period. Breeding conditions were maintained as a constant temperature and humidity of 20°C±2°C and 60% with a 12-hr day and 12-hr night period.
We performed random sampling and divided the animals into following four groups (n=5): nonexercising male and normal female group, exercising male and normal female group, nonexercising male and obese female group, and exercising male and obese female group. Only male rat pups (4 weeks old) were used for the experiment. The rat pups from the above groups were redivided into following four groups (n=10): paternal nonexercised male pups from normal maternal rats, paternal exercised male pups from normal maternal rats, paternal nonexercised male pups from obese maternal rats, and paternal exercised male pups from obese maternal rats.
BrdU (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) was given intraperitoneally to all animals 1 hr before the starting of the treadmill exercise for 3 consecutive days prior to sacrifice.
Exercise protocols
The rats in the exercise groups performed treadmill running. Exercise consisted of 5 min of warm up at a 0° inclination at 3 m/min, 30 min of the main exercise at 10 m/min, and 5 min of cool down at 3 m/min or the first 3 weeks. Following this regimen, 40 minutes of the main exercise at 10 m/min during 4–6 weeks, 30 min of the main exercise at 15 m/min during 7–9 weeks, and 40 min of the main exercise at 15 m/min during final 10–12 weeks were performed. The exercise was performed once a day and 6 days per week during 12 consecutive weeks.
Morris water maze task
Spatial learning ability was evaluated using the Morris water maze task. This task requires rats to learn the spatial location of a hidden platform in a black circular pool (180 cm in diameter and 50 cm high) filled with clear water (25°C±1°C). The hidden platform (15 cm in diameter and 40 cm high) was placed 2 cm below the surface of water in the middle of the north quadrant and was camouflaged by virtue of being transparent against a black background. Distal visual cues were placed on the walls around the pool. The position of the cues remained unchanged through the task. One day before training, the rat pups were habituated to swimming for 60 sec in the pool without a platform. All rat pups were trained three times a day for four consecutive days before sacrifice.
Probe trail was conducted 24 hr after the last training. When finding the platform, the rat pups were allowed to remain for 30 sec. If the rat pups did not find the platform within 60 sec, they were guided by hand to the platform. The rat pups were given 60 sec retention probe test, and then the platform removed from the pool. Data were automatically collected via the Smart Video Tracking System (Smart ver. 2.5, Panlab, Barcelona, Spain).
Tissue preparation
The animals were sacrificed immediately after Morris water maze task. The animals were fully anesthetized with diethyl ether, then transcardially perfused with 50-mM phosphate-buffered saline (PBS) followed by freshly prepared 4% paraformaldehyde in 100-mM phosphate buffer (pH, 7.4). The brains were then removed, postfixed in the same fixative overnight, and transferred to a 30% sucrose solution for cryoprotection. Coronal sections of 30-μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
Immunohistochemistry for BrdU
The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2X standard saline citrate at 65°C for 2 hr, denatured in 2 N HCl at 37°C for 30 min, and rinsed twice in 100-mM sodium borate (pH, 8.5). The sections were then incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 hr. The sections were then incubated for 1 hr with an ABC complex (1:100; Vector Laboratories). For visualization, the sections were incubated in 50-mM Tris-HCl (pH, 7.6) containing 0.03% diaminobenzidine (DAB), 40-mg/mL nickel chloride, and 0.03% hydrogen peroxide for 5 min.
After BrdU labeling, a mouse anti-neuronal nucleic antibody (1:1,000; Chemicon International, Temecula, CA, USA) was used on the same sections to differentiate neurons. The sections were washed 3 times with PBS, incubated for l hr with a biotinylated anti-mouse secondary antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.03% DAB and 0.03% hydrogen peroxide for 5 min. The sections were mounted onto gelatin-coated slides, air-dried overnight at room temperature, and covers lipped under Permount (Thermo Fisher Scientific Inc., Waltham, MA, USA).
Immunohistochemistry for DCX
The sections were incubated in PBS for 10 min, washed three times in PBS, then incubated in 1% hydrogen peroxide for 30 min. The sections were incubated overnight with goat anti-DCX antibody (1:1,000; Oncogene Research Product, Cambridge, UK). They were then incubated with the appropriate biotinylated secondary antibody (1:200; Vector Laboratories) for another 1 hr, washed, and incubated in ABC complex (Vector Elite ABC kit; 1:100; Vector Laboratories). Labeling was visualized using 0.03% DAB, and the sections were mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and the coverslips were mounted using Permount (Thermo Fisher Scientific Inc.).
Western blotting for BDNF and TrkB
Collected hippocampal samples were homogenized on ice and lysed in a lysis buffer containing 50-mM Tris-HCl (pH, 7.5), 150-mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% sodium dodecyl sulfate (SDS), 1-mM phenylmethylsulfonyl fluoride, and 100-mg/mL leupeptin. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Thirty micrograms of total protein was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. The membrane was blocked with dehydrated milk, then incubated with mouse anti-β-actin antibody (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-BDNF/TrkB antibody (1:1,000; Santa Cruz Biotechnology). After washing, horseradish peroxidase-conjugated, species appropriate secondary antibodies were applied. Incubations were performed at room temperature. The bands were detected using the enhanced chemiluminescence detection system (Santa Cruz Biotechnology), and quantified using an Image-Pro Plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA).
Statistical analyses
Cell counting and optical density quantification were performed using Image-Pro Plus (Media Cyberbetics Inc.) attached to a light microscope (Olympus, Tokyo, Japan). The data were analyzed with one-way analysis of variance, followed by Duncan post hoc tests. All values are expressed as the mean±standard error of the mean, and P<0.05 was considered significant.
RESULTS
Effect of paternal treadmill exercise on spatial learning ability in male rat pups
To assess spatial learning ability, we performed a Morris water maze task (Fig. 1). From the assessments performed on day 2, 3, and 5, the male rat pups from the obese maternal rats showed reduced spatial learning ability compared to the male rat pups from the normal maternal rats. Paternal exercise improved spatial learning ability in the male rat pups from the obese maternal rats.

Effect of paternal treadmill exercise on spatial learning ability in male rat pups. Left panel: Swimming pathway. Middle panel: Daily escape latency in Morris water maze task. Right panel: Time in probe quadrant in Morris water maze task. A, paternal nonexercised male pups from normal maternal rats; B, paternal exercised male pups from normal maternal rats; C, paternal nonexercised male pups from obese maternal rats; D, paternal exercised male pups from obese maternal rats. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the paternal nonexercised male pups from normal maternal rats. #P<0.05 compared to the paternal nonexercised male pups from obese maternal rats.
Effect of paternal treadmill exercise on BDNF and TrkB expressions in male rat pups
BDNF and TrkB expressions were quantified by western blotting (Fig. 2). Expressions of BDNF and TrkB in the hippocampus were lower in the male rat pups from the obese maternal rats compared to the male rat pups from the normal maternal rats. Paternal exercise enhanced BDNF and TrkB expressions in the male rat pups from the obese maternal rats.
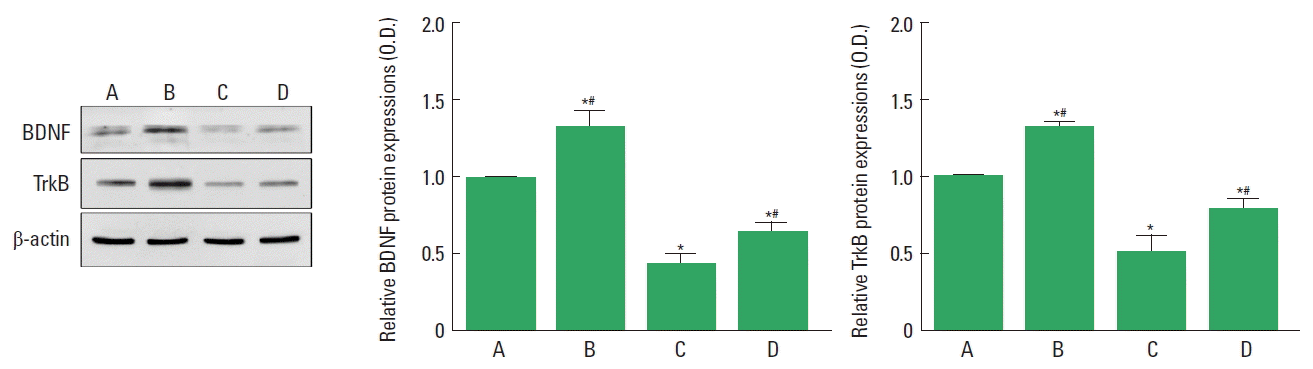
Effect of paternal treadmill exercise on hippocampal brain-derived neurotrophic factor (BDNF) and tyrosine kinase B receptor (TrkB) expressions in male rat pups. Left panel: Western blot of BDNF and TrkB expressions. Middle & right panel: BDNF and TrkB expressions in each group. A, paternal nonexercised male pups from normal maternal rats; B, paternal exercised male pups from normal maternal rats; C, paternal nonexercised male pups from obese maternal rats; D, paternal exercised male pups from obese maternal rats. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the paternal nonexercised male pups from normal maternal rats. #P<0.05 compared to the paternal nonexercised male pups from obese maternal rats.
Effect of paternal treadmill exercise on cell differentiation in male rat pups
We used DCX labeling to assess cell differentiation in the hippocampus (Fig. 3). Cell differentiation in the hippocampus was decreased in the male rat pups from the obese maternal rats compared to the male rat pups from the normal maternal rats. Paternal exercise increased cell differentiation in the male rat pups from the obese maternal rats.

Effect of paternal treadmill exercise on hippocampal cell differentiation in male rat pups. Upper panel: Photomicrographs of DCX-positive cells. The scale bar represents 100 μm. Lower panel: The number of DCX-positive cells in each group. A, paternal nonexercised male pups from normal maternal rats; B, paternal exercised male pups from normal maternal rats; C, paternal nonexercised male pups from obese maternal rats; D, paternal exercised male pups from obese maternal rats; DCX, doublecortin. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the paternal nonexercised male pups from normal maternal rats. #P<0.05 compared to the paternal nonexercised male pups from obese maternal rats.
Effect of paternal treadmill exercise on cell proliferation in male rat pups
We used BrdU labeling to assess cell proliferation in the hippocampus (Fig. 4). Cell proliferation in the hippocampus was decreased in the male rat pups from the obese maternal rats compared to the male rat pups from the normal maternal rats. Paternal exercise increased cell proliferation in the male rat pups from the obese maternal rats.
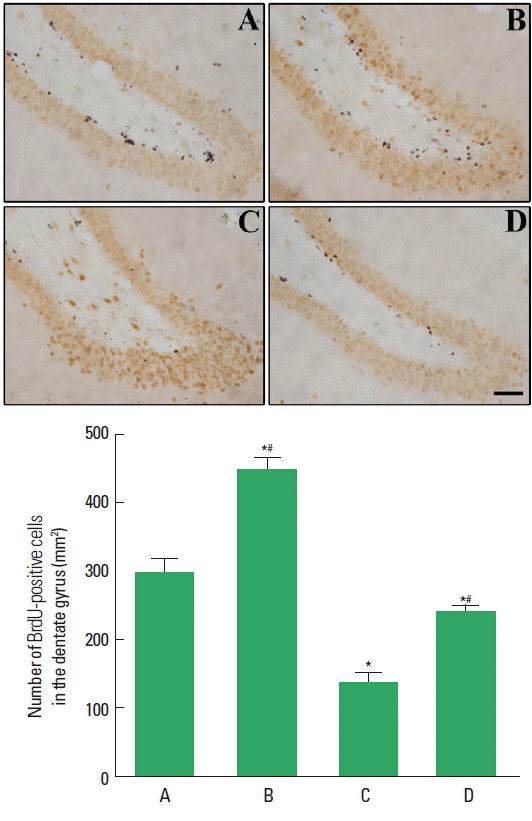
Effect of paternal treadmill exercise on hippocampal cell proliferation in male rat pups. Upper panel: Photomicrographs of BrdU-positive cells. The scale bar represents 100 μm. Lower panel: The number of BrdU-positive cells in each group. A, paternal nonexercised male pups from normal maternal rats; B, paternal exercised male pups from normal maternal rats; C, paternal nonexercised male pups from obese maternal rats; D, paternal exercised male pups from obese maternal rats; BrdU, 5-Bromo-2′-deoxyuridine. Data are expressed as the mean±standard error of the mean. *P<0.05 compared to the paternal nonexercised male pups from normal maternal rats. #P<0.05 compared to the paternal nonexercised male pups from obese maternal rats.
DISCUSSION
Maternal nutrients exerted significant effect on the development and maintenance of brain function of offspring (Tozuka et al., 2009a). Maternal high-fat diet provided a hypothalamic leptin resistance in offspring, however, fails to increase the body weight gain until adulthood (Férézou-Viala et al., 2007). C57BL/6 mice received high-fat diet for 6 weeks, and reduced expression of BDNF, disturbed spatial cognitive function, increased lipid peroxidation, and reduced neurogenesis were observed in the offspring born from the obese mother (Tozuka et al., 2009b; Tozuka et al., 2010).
In the present study, obese maternal rats were mad by feeding with high-fat diet for 18 weeks. Spatial learning ability in the Morris water maze task was reduced in the male rat pups born from the obese maternal rats. Expressions of BDNF and TrkB in the hippocampus were suppressed in the male rat pups born from the obese maternal rats. Cell proliferation and differentiation in the hippocampus were reduced in the male rat pups born from the obese maternal rats.
Exercise increases neurogenesis in the hippocampus, and exercise is also known to improve hippocampus-dependent learning ability and memory function (Cotman and Berchtold, 2002; Vaynman et al., 2007). The enhancing effect of treadmill exercise on spatial learning ability could be ascribed to the increment of neurogenesis (Kim et al., 2013b).
Paternal obesity increases damage to sperm DNA, as a result, reduced the rate of pregnancy and birth (Bakos et al., 2011). Paternal age and nutrition influence the development of offspring (Curley et al., 2011) and paternal environment also exert impact on the brain development of offspring (Mychasiuk et al., 2012). Exercise improved metabolic status and sperm function in obese men (Palmer et al., 2012). Paternal treadmill exercise enhanced hippocampus-dependent spatial learning and memory in the male pups (Yin et al., 2013).
In the present study, paternal treadmill exercise improved spatial learning ability in the male rat pups born from the obese maternal rats. Paternal treadmill exercise increased BDNF and TrkB expressions in the male rat pups born from the obese maternal rats. Cell proliferation and differentiation in the male rat pups born from the obese maternal rats were facilitated by paternal treadmill running. These results suggest that paternal exercise enhanced hippocampal neuroplasticity and consequently improved spatial learning ability in the rat pups born of the obese maternal rats.
Based on the present results, it can be concluded that maternal obesity deteriorates brain function of offspring, in contrast, paternal exercise improves brain function through enhancing neuroplasticity of offspring born of obese mother.
ACKNOWLEDGMENTS
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2014S1 A5B5A07041321).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
