Association study between growth hormone receptor (GHR ) gene polymorphisms and obesity in Korean population
Article information
Abstract
A main target of growth hormone (GH) is adipose tissue in human body. The GH secretion in obesity patients is impaired. It is needless to say that growth hormone receptor (GHR) is necessary in GH hormone signaling. The purpose of the present study is to examine the association between single nucleotide polymorphisms (SNPs) and the development of obesity. A total of 211 overweight/obese subjects with a body mass index (BMI) ≥23 kg/m2 and 157 nonoverweight/obese controls with a BMI of 18.5–23.0 kg/m2 were involved in this study. Seven SNPs including the rs6451620 (intron), rs4130114 (intron), rs4410646 (intron), rs6898743 (intron), rs4394131 (intron), rs6182 (Cys440Phe), and rs6184 (Pro579Thr) and rs2229765 SNPs of GHR gene were genotyped. Genotyping was performed using custom DNA chip. SNPStats was used to calculate the odds ratio, 95% confidence interval, and P-value. The link-age disequilibrium block and haplotypes among seven SNPs were determined using Haploview version 4.2. Dominant, recessive, and log-additive genetic models were conducted for genetic analyzing. Among tested SNPs in GHR gene, rs4410646 and rs6898743 showed significant association with obesity (rs4410646, P=0.02 in dominant model and P=0.036 in log-additive model; rs6898743, P=0.039 in dominant model and P=0.044 in log-additive model). In summary, these results suggest that GHR gene polymorphisms might play a role in the development of obesity in the Korean population.
INTRODUCTION
Obesity is a severe social problem in Korea (Kang, 2013). Not only social and economic factors (Chung et al., 2016) but also other various risk factors are important factors affecting the pathophysiologic and developing process of obesity. And life style and psychological factors are also related to hormone changes in obesity (Azuma et al., 2015; Kim et al., 2016; Nordkap et al., 2016).
Growth hormone (GH) is affected by psychological stress and many physiological stresses (He et al., 2015; Karaca et al., 2016). And GH action is related to body energy expenditure and body weight (Glad et al., 2015; Haruta et al., 2015; Liu et al., 2016). It is a major hormone in insulin resistance and body weight control. Therefore, GH is widely studied in many field of studies (Huang et al., 2016; Imran et al., 2016; Karaca et al., 2016).
However, hormone signaling of GH needs growth hormone receptor (GHR) (Carter-Su et al., 2016) because GH is not a steroid hormone (Carter-Su et al., 2016), and its main function is related to signaling of GHR into a cell. GHR deficiency is related with obesity, diabetes and cancer risk is likely appears. Therefore deficient GHR may affect GH function to be less modulation signals. It may show a kind of decreased pituitary hormone situation, which cause higher risk of metabolic syndrome (Khang et al., 2016), because the studies suggest that GH function is related with GHR function. GHR deficiency is related with adiposity. One of the famous example is GHR-null (GHR[−/−]) mouse (Sackmann-Sala et al., 2014), which showed insulin sensitivity and increased adiposity.
Insulin sensitivity is another problem in obesity, and GH is a major hormone in insulin sensitivity (Galescu et al., 2016; Poggiogalle et al., 2016; Suda et al., 2016). GH deficiency may lead to obesity, fatty liver, and sarcopenia. Those are closely related keywords to obesity, and these studies suggest that polymorphisms of GHR gene may associate with obesity. However, genetic effect such as common variation of functional gene may also affect its function (Barrie et al., 2017; Rahim et al., 2016). Therefore, in this study, we investigated the relationship between genetic polymorphisms of GHR gene and obese vs. nonobese in a Korean population, to compare polymorphism effect of GHR on obesity.
MATERIALS AND METHODS
Study subjects
Three hundred sixty-eight subjects who participated in a general health check-up program were enrolled in this study. The biochemical characteristics including fasting plasma glucose, fasted glycated hemoglobin, total cholesterol, and low-density lipoprotein cholesterol were measured. The values of biochemical test are shown in Table 1. Body mass index (BMI) is calculated based on the weight (kg) divided by the square of height (m) (Yang, 2015). All subjects were classified according to the classification of Korean Society for the Study of Obesity (underweight, BMI<18 kg/m2; normal, BMI 18 to <23 kg/m2; moderately obese, BMI 23 to <25 kg/m2; obesity I, BMI 25 to <30 kg/m2; obesity II, BMI≥30 kg/m2). Two hundred eleven subjects with a BMI≥23 kg/m2 were included in overweight/obese group (mean age±standard deviation, 44.7±6.4 years) and 157 subjects with a BMI of 18.5–23.0 kg/m2 in nonoverweight/obese control group (mean age±standard deviation, 43.6±6.2 years).
SNP selection and genotyping
Genomic DNAs were extracted from peripheral bloods of all subjects by Roche DNA extraction kit (Roche Diagnostics Corp., Indianapolis, IN, USA) and quantified using nanodrop machine. We selected seven SNPs including the rs6451620 (intron), rs4130114 (intron), rs4410646 (intron), rs6898743 (intron), rs4394131 (intron), rs6182 (Cys440Phe), and rs6184 (Pro579Thr) and rs2229765 in GHR gene. Each SNP was genotyped using custom DNA chip. After that, each genotype of SNPs was analyzed using targeted genotyping analysis software GCOS software (Affymetrix).
Statistical analysis
To evaluate the odds ratio (OR), 95% confidence interval (CI), and P-value, SNPStats (http://bioinfo.iconcologia.net/index.php) and IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA) were used. Genetic models (dominant [major homozygous vs. heterozygous+minor homozygous], recessive [major homozygous+heterozygous vs. minor homozygous], and log-additive [major homozygous vs. heterozygous vs. minor homozygous] models) were applied (Kim et al., 2014; Zare et al., 2013). The haplotype analysis in linkage disequilibrium (LD) was performed using Haploview 4.2. The P-value less than 0.05 was considered as statistically significant.
RESULTS
To find genetic factors involved in the development of obesity, we performed an association study between polymorphisms of GHR gene and overweight/obese. Firstly, we calculated Hardy-Weinberg equilibrium (HWE) in tested polymorphisms of GHR gene. The genotype distributions of the tested seven SNPs of GHR gene were consistent with the HWE (rs6451620, P=1.00; rs4130114, P=1.00; rs4410646, P=0.16; rs6898743, P=1.00; rs4394131, P=1.00; rs6182, and rs6184, P=1.00; rs2229765, P=1.00, data not shown).
Tables 2 and 3 showed genotype and allele distributions of seven SNPs in GHR gene. Among tested seven SNPs of GHR gene, two SNPs (rs4410646 and rs6898743) revealed the significant association with obesity (rs4410646, OR, 1.68, 95% CI, 1.09–2.60, P=0.02 in dominant model [A/A genotype vs. A/C genotype+C/C genotype] and OR, 1.38, 95% CI, 1.02–1.87, P=0.036 in log-additive model [A/A genotype vs. A/C genotype vs. C/C genotype] rs6898743, OR, 0.63, 95% CI, 0.41–0.98, P=0.039 in dominant model [C/C genotype vs. C/G genotype+G/G genotype] and OR, 0.73, 95% CI, 0.54–0.99, P=0.044 in log-additive model [C/C genotype vs. C/G genotype vs. G/G gentoype]).

Genotype analysis of polymorphisms in growth hormone receptor (GHR ) gene between overweight/obesity and control subjects
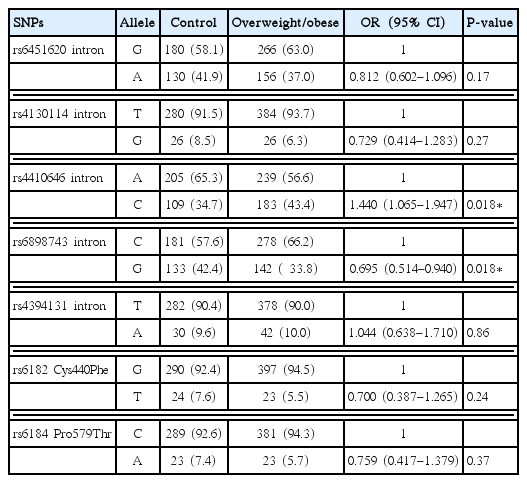
Allele analysis of polymorphisms in growth hormone receptor (GHR ) gene between overweight/obesity and control subjects
However, the other five SNPs did not show any statistical significant association with susceptibility of obesity.
In haplotype analysis, we found two haplotypes (GC and TA haplotype) of one LD block in GHR gene, which consisted of rs6182 and rs6184. But two haplotypes of GHR gene showed no statistical significant difference between the control group and the overweight/obese group (GC haplotype, P=0.22 and TA haplotype, P=0.30) (Table 4).
DISCUSSION
In present study, seven SNPs in GHR gene were studied, however, only two intron SNPs of them were weakly associated with obesity (rs4410646 and rs6898743). The two SNPs were not similar in major/minor allele frequencies, however, both of them showed association to obesity in the dominant and log-additive models, and not in recessive model. Among the other SNPs, four SNPs (rs4130114, rs4394131, rs6182, and rs6184) displayed similar major/minor allele frequencies, and all of them showed no association with obesity.
Association between rs4410646 SNP of GHR gene and obesity was previously found in previous study (Mong et al., 2010). They reported that rs4410646 was significantly associated with the body composition (P=0.0044) and blood pressure factor scores (P=0.00017) (Mong et al., 2010). They also found rs7703713 SNP may affect IGF-1 activity factor score. However, the SNP was not included in our study. Interestingly, in their study, C genotype was associated with less obesity. It was on the contrary to our study. Our result was that C genotype was associated with overweight/obese group.
In addition, the association between rs6898743 SNP of GHR gene and cancer were studied, and rs6898743 was associated with esophageal adenocarcinoma (McElholm et al., 2010; Ong et al., 2014). But there was no obesity study was found.
In general, our result may show only weak association of GHR SNPs and obesity susceptibility. However, some presumable results may support our results. A variation in GHR gene was studied in various anthropometry (Glad et al., 2015), and relationship to central obesity was found. However, in their discussion, deficit in GHR does not always directly show GH deficiency, because they found responses to exogeneous GH in deficient GHR. They suggested the complex and continuous lifelong relationship among GH sensitivity, GH secretion, and other metabolic mechanisms in normal GH–IGF1 axis.
Another example of complex GH sensitivity may be the white adipose tissue of GHR-null mice (Sackmann-Sala et al., 2014). They presented the insulin, leptin, adiponectin, and protein changes in enhanced insulin sensitivity (Sackmann-Sala et al., 2014). In addition, Beattie et al. (2002) reported that decreased binding affinity may not reflect biological activities in the protein of living organism.
To our knowledge, this is the first study to report the relationship between SNPs in the GHR gene and obesity by BMI. Our findings support that GHR may play a role in pathophysiologic signaling of obesity or other related diseases. And further study in a large numbers with more anthropometry will be needed to verify our findings.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.

