Matrix metalloproteinase and tissue inhibitor of metalloproteinase responses to muscle damage after eccentric exercise
Article information
Abstract
High-intensity eccentric exercise is known to induce muscle damage leading to inflammatory responses and extracellular matrix (ECM) degradation. These degradation processes involve enzymes such as matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs). MMPs are calcium and zinc-dependent proteolytic enzymes that play a role in ECM degradation and recruitment of inflammatory and myogenic cells into the damaged site. In contrast, TIMPs inhibit MMP-induced ECM degradation to maintain normal homeostasis in ECM. Recently, several studies have examined the process of muscle remodeling and the roles of ECM, MMPs, and TIMPs in exercise-induced muscle damage. However, the results of these studies are not inconsistent. In the present mini-review, we will discuss the responses of MMP and TIMP to eccentric exercise based on the literature review.
INTRODUCTION
High-intensity eccentric exercise is known to induce muscle damage (Clarkson and Hubal, 2002). Morphologically, muscle damage leads to cell membrane disruption (Nosaka et al., 2006), infiltration of inflammatory cells, such as neutrophils and macrophages, into the site of damage (Clarkson and Hubal, 2002; Tidball, 2005), and degradation of the extracellular matrix (ECM) (Kjaer et al., 2006). Fragments resulting from ECM degradation facilitate the mobilization of cells related to the inflammatory response and increase chemotactic activity and phagocytosis, ultimately playing a role in the development of delayed onset muscle soreness (Stauber et al., 1990). Increased ECM degradation following muscle damage has also been reported to reduce force transmission (Gao et al., 2008). However, ECM degradation also facilitates the movement and regeneration of satellite cells (Chen and Li, 2009).
Several enzymes play important roles in ECM degradation; of these enzymes, matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs) have been extensively studied (Kieseier et al., 2001). MMPs have been reported to degrade ECM (Mu et al., 2010; Rullman et al., 2013), whereas TIMPs inhibit the enzymatic functions of MMPs (Alameddine, 2012). Excessive activation of MMPs increases tissue degradation, hindering myogenesis (Kieseier et al., 2001). Hence, the balance between MMPs and TIMPs must be well managed to optimize postdamage ECM remodeling.
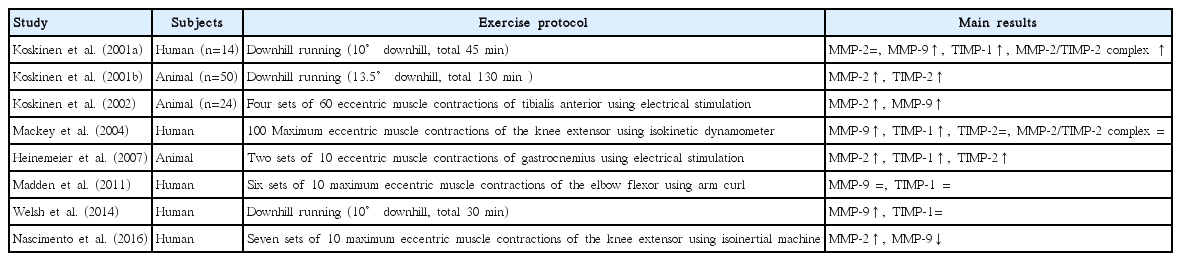
A few studies have examined MMP and TIMP responses following eccentric exercise. Koskinen et al. (2002) reported that high-intensity eccentric muscle contraction significantly increases MMP-2 and MMP-9 expression. Similarly Mackey et al. (2004) reported that MMP-9 and TIMP-1 levels are significantly increased following eccentric exercise. In contrast, Nascimento et al. (2016) reported that MMP-2 and -9 levels actually decrease after eccentric exercise, whereas Madden et al. (2011) reported that MMP-9 and TIMP-1 levels remain unchanged after eccentric exercise.
Although recent studies of exercise-induced muscle damage have focused on MMP and TIMP responses following eccentric exercise, the importance of ECM degradation regulated by MMP and TIMP during recovery from muscle damage is not well recognized. In fact, MMP and TIMP responses have been documented in the field of cancer and chronic inflammatory diseases. Therefore, this mini-review will discuss MMP and TIMP responses to muscle damage following eccentric exercise based on the previous literatures.
MATRIX METALLOPROTEINASE IN SKELETAL MUSCLE
Skeletal muscle comprises contractile myofibrils and ECM (Gao et al., 2008). ECM is involved in the maintenance of normal cell physiology, including structural integration, proliferation, differentiation, and apoptosis (Gattazzo et al., 2014). Furthermore, ECM supports the structure of the skeletal muscle by enhancing mechanical stress and plays a critical role in force transmission (Koskinen et al., 2002). MMPs are calcium and zinc-dependent proteolytic enzymes, also known as matrixins (Nagase et al., 2006). About 24 MMPs have been identified in humans (Klein and Bischoff, 2011); these 24 MMPs can be subdivided into membrane-type MMPs and soluble-type MMPs (Barnes et al., 2009). MMP collagenases (MMP-1, -8, -13, and -18) break down interstitial collagen types I, II, and III and MMP gelatinases (MMP-2 and -9) degrade denatured collagen type IV, VII, and X (Monaco et al., 2006). Collagen type IV is a key component of basement membranes as well as cell arrangement in tissues (Carmeli et al., 2004).
MMP can degrade ECM alone or in combination with the plasminogen/plasmin system to facilitate the migration of inflammatory and myogenic cells, such as satellite cells, from ECM to the site of injury (Mann et al., 2011). During the process of ECM degradation, several cytokines and growth factors are also released to regulate the proliferation and differentiation of the migrated cells (Lu et al., 2011). This process is required for the remodeling of the tissue and is crucial for maintaining skeletal muscles in a normal state. An in vitro study showed that the migration of satellite cells to the injury site is a key process in the regeneration of skeletal muscles and that ECM degradation can be a pivotal component of satellite cell migration and regeneration (Chen and Li, 2009). In particular, MMP-2 and -9 show biological activities mostly in skeletal muscles because their primary substrate is a key component of ECM in skeletal muscles (Sternlicht and Werb, 2001). MMP-2 and -9 have been reported to be expressed in different stages; MMP-2 may be activated simultaneously with the regeneration of muscle fibers, whereas MMP-9 may be expressed in association with the early inflammatory response and activation of satellite cells (Agren, 1994; Carmeli et al., 2004; Kherif et al., 1999). Taken together, these findings suggest that MMP-2 and -9 are involved in not only inflammatory responses but also ECM remodeling and muscle regeneration. Clinical studies on pathophysiology have reported that hyperactivation of MMP facilitates the infiltration and metastasis of tumor cells, which may induce carcinogenesis, and may cause chronic diseases, such as fibrosis, rheumatoid arthritis, and Parkinson’s disease (Ramezani and Shamsara, 2015).
TISSUE INHIBITOR OF METALLOPROTEINASE IN SKELETAL MUSCLE
TIMPs reduce excessive MMP-induced ECM degradation (Alameddine, 2012). A 1:1 ratio between MMPs and TIMPs prevents hyperactivation of MMPs (Visse and Nagase, 2003). There are four TIMP isoforms (i.e., TIMP-1, -2, -3, and -4), which show tissue-specific, constitutive, or inducible expression depending on the transcriptional level. TIMP-1 is widely expressed in many mammalian tissues induced by growth factors, phorbol esters, and cytokines. TIMP-2 is constitutively expressed in most tissues. TIMP-3 is expressed in the heart, kidneys, and thymus. Finally, TIMP-4 has tissue-specific functions, commonly expressed in the heart, kidneys, lungs, testes, and brain, but rarely expressed in muscles and ovaries (Murphy, 2011). Among the four TIMP isoforms, TIMP-1 and -2 have been reported to be significantly upregulated in eccentric exercise-induced muscle damage (Koskinen et al., 2002) or rotator cuff tears (Castagna et al., 2013).
MATRIX METALLOPROTEINASE AND TISSUE INHIBITOR OF METALLOPROTEINASE RESPONSES TO ECCENTRIC EXERCISE
Eccentric exercise induces muscle damage, increases mobilization of inflammatory cells (Clarkson and Hubal, 2002). As shown in Fig. 1, ECM responds to forceful mechanical stress, such as high-intensity eccentric exercise (Gao et al., 2008; Kovanen, 2002; Mackey et al., 2004), during which ECM is degraded as muscle fiber damage occurs (Stauber, 2004). Additionally, MMP and TIMP responses occur simultaneously (Mackey et al., 2004). MMPs, specifically MMP-2, are downregulated during low-intensity exercise, but upregulated in high-intensity exercise (Carmeli et al., 2005); some studies have reported that the expression and activation of MMP-2 or -9 are increased following high-intensity eccentric exercise (Koskinen et al., 2001a, 2002; Mackey et al., 2004). Koskinen et al. (2001a) reported that eccentric exercise via downhill running results in acute increases in MMP-9, TIMP-1, and the MMP-2/TIMP-2 complex. In addition, Mackey et al. (2004) reported that high-intensity eccentric exercise on a knee extensor results in an increase in MMP-9 until eight days after exercise and an increase in TIMP-1 for four days after exercise and continued to increase up to 14 days. Similar results were found after eccentric muscle contractions via electrical stimulation. Heinemeier et al. (2007) found that eccentrically biased electrical stimulation increases MMP-2, TIMP-1, and TIMP-2 expression while Koskinen et al. (2002) suggested that the electrical stimulation in mice significantly increases the mRNA expression and activation of MMP-2 and -9 between 4 and 7 days following exercise.

MMP and TIMP responses after eccentric exercise. ECM, extracellular matrix; GH, growth hormone; MMP, matrix metalloproteinase; TIMP, tissue inhibitor of metalloproteinase.
Muscle damage may lead to an increase in the number of leukocytes and prostaglandin concentrations (Prisk and Huard, 2003). Leukocytes include inflammatory cells, such as neutrophils and macrophages (Tidball, 2005); prostaglandin facilitates inflammatory responses and is released by high-intensity muscle contractions (Karamouzis et al., 2001). MMP-9 is synthesized by leukocytes (Murphy et al., 1989), and prostaglandin stimulates the expression of MMP-2 and -9 in human T cells (Goetzl et al., 1996). In fact, Koskinen et al. (2002) have microscopically observed the infiltration of inflammatory cells in injured muscles with increased MMP-9 levels following eccentric muscle contractions. In addition, some studies have reported that ECM degradation assists the migration and invasion of immune cells into the injured muscle (Carmeli et al., 2004; Klein and Bischoff, 2011). Parks and Mecham (2011) suggested that MMP is a biological marker representing the inflammatory response.
Koskinen et al. (2001a) reported that after downhill running, TIMP-1 is expressed in the early stages of muscle damage, whereas TIMP-2 is expressed during the later stages of muscle damage. TIMP-1 is intimately related to MMP-9, and TIMP-2 binds strongly to MMP-2 (Goldberg et al., 1989). Some studies have shown that the activity levels of TIMPs correspond to changes in the activity of MMP-2 and -9 (Overall et al., 1991; Singh et al., 2000). As previously described, MMP-2 is usually activated during the early stages of muscle regeneration, whereas MMP-9 is typically activated during the early stages of the inflammatory response. Thus, MMP-9 and TIMP-1 may interact during the early stages of exercise-induced muscle damage, and MMP-2 and TIMP-2 may interact during the later stages of muscle damage (muscle regeneration). In essence, MMP-9/TIMP-1 and MMP-2/TIMP-2 have antagonistic effects in the inflammatory response following eccentric exercise and regulate the degradation and remodeling of ECM.
However, there is little evidence to support the relationship between posteccentric exercise MMP and TIMP responses and inflammatory cells. Most existing studies have produced limited results due to the use of animal models. Recently, Nascimento et al. (2016) reported that acute eccentric exercise reduces the levels of MMP-2 and -9 up to 48 hr following exercise and interleukin-6 levels were also reduced. These data suggest that MMP-2 and -9 levels may be altered in parallel to changes in the expression of inflammatory factors. However, Nascimento et al. (2016) studied obese women, whereas most previous studies of eccentric exercise have been conducted in healthy subjects. Obese people are known to have an imbalance in inflammatory factors (Jung and Choi, 2014). Nascimento et al. (2016) also demonstrated that exercise may result in anti-inflammatory responses by reducing MMP-2 and -9 levels because obese individuals maintain a high inflammatory response, even at rest, as compared with healthy subjects. Therefore, future studies should also examine changes in and the relationships among MMP-2, -9, and inflammatory factors following eccentric exercise in healthy subjects.
Nonetheless, some studies have suggested that eccentric exercise does not induce significant MMP or TIMP responses, and some researchers have argued that there is no association between the two. Welsh et al. (2014) reported that although MMP-9 is significantly increased after downhill running, the change is not substantial. Madden et al. (2011) reported that there were no changes in MMP-9 and TIMP-1 responses at all time points following eccentric exercise using an elbow flexor model. Unlike prior studies, which mainly used the downhill running model, Madden et al. (2011) examined MMP responses after exercise using an elbow flexor model. The elbow flexor model is known to induce greater muscle damage than the downhill running model (Jamurtas et al., 2005). Despite such differences in exercise models, there were no changes in MMP-9 after eccentric exercise using an elbow flexor model. This result may indicate that eccentric exercise using an elbow flexor model does not involve the muscles sufficiently for MMP-9 response (Madden et al., 2011). Madden et al. (2011) suggested that differences in the exercise protocol may also explain these discrepancies. As shown in Table 1, many studies consistently found MMP and TIMP responses following downhill running and knee extensor models (Koskinen et al., 2001a; Mackey et al., 2004). Likewise, MMP-9 levels seems to show greater increase during whole body exercise or exercise with large muscle groups involved, such as marathon and cycling compared to local exercise with small volume of muscles involved such as arm curls (Rullman et al., 2007; Saenz et al., 2006). This may be supported by differences in ECM composition, turnover, systemic vasculature, and local clearance between upper and lower muscles (Madden et al., 2011); however, it has yet to be scientifically verified. Since the study conducted by Madden et al. (2011) remains the only study using an elbow flexor model, additional studies using similar models are required to produce meaningful conclusions.
CONCLUSIONS
Eccentric exercise induces muscle damage and increases ECM degradation, during which MMPs and TIMPs are also activated. These two enzymes maintain the homeostasis of ECM via antagonistic effects in muscle degradation and remodeling. To date, MMP and TIMP responses have mostly been studied in animals or patients with chronic illnesses. Furthermore, studies on muscle damage have also been conducted by inducing damage by freeze exposure or drug injections rather than exercise. Although some studies have examined posteccentric exercise MMP and TIMP responses, only animal studies have produced clear MMP and TIMP responses following eccentric muscle contraction. Studies in human subjects have produced conflicting results. This may be due to differences in the subjects and exercise protocols. More studies on human subjects, particularly using elbow flexor models, should be conducted and posteccentric exercise inflammatory responses or factors related to muscle regeneration should be measured in addition to MMP and TIMP responses. In addition, because ECM degradation may reduce force transmission, studies should analyze the association between posteccentric exercise MMP or TIMP levels and reduction of maximum voluntary muscle contraction.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.