Impact of aerobic and anaerobic exercise training on oxidative stress and antioxidant defense in athletes
Article information
Abstract
Exercise mediates an excessive free radical production leading to oxidative stress (OS). The body has natural antioxidant systems that help decrease OS, and these systems may be enhanced with exercise training. However, only a few studies have investigated the differences in resting OS and antioxidant capacity (AOC) between aerobically trained athletes (ET), anaerobically trained athletes (RT), and untrained individuals (UT). Therefore, this study sought to investigate the resting and postexercise OS and AOC in ET, RT, and UT. Sixty healthy young males (26.6±0.8 yr) participated in this study. Subjects were divided into three groups, ET, RT, and UT by distinct training background. Resting plasma malondialdehyde (MDA) and protein carbonyls (PC) were not significantly different in ET, RT, and UT. However, MDA and PC were significantly increased following a graded exercise test (GXT) in UT but not in ET and RT. Resting total antioxidant capacity (TAC) levels and TAC were not different in ET, RT, and UT. Interestingly, TAC levels significantly decreased after the GXT in all groups. Additionally, UT showed lower post-exercise TAC levels compared to ET and RT. These results showed that ET, RT, and UT have similar OS and AOC at rest. However, both ET and RT have greater AOC against exercise mediated OS compared to UT. These findings may explain, at least in part, why both aerobic and anaerobic types of exercise training improve redox balance. However, it appears there is no specific exercise type effect in terms of redox balance.
INTRODUCTION
The oxidative activity of reactive oxygen species (ROS) is a normal function of cellular respiration, but can impair the normal functioning of other molecules and systems if present in high amounts (McBride and Kraemer, 1999). The body has natural antioxidant systems that help to keep ROS levels normal, however, these antioxidant systems can be overwhelmed, causing an acute accumulation of ROS known as oxidative stress (OS) (McBride and Kraemer, 1999; Sen et al., 1994). Imbalance between OS and the body’s own antioxidant system has been implicated in exercise fatigue, loss of function with aging, and several disease processes (Alessio, 2000; Clarkson and Thompson, 2000; Lambert and Yang, 2003; McBride and Kraemer, 1999; Sen and Packer, 2000).
Exercise is a potent producer of ROS and research has demonstrated that increased exposure to ROS triggers an adaptation in antioxidant enzyme activity in cardiac and skeletal muscle tissues in animal models (Poulsen et al., 1999). Also, there is clear evidence that chronic exercise training improves antioxidant capacity (AOC) (Ji, 1999). In addition, studies suggest that aerobic endurance training can increase the AOC of untrained individuals (Mena et al., 1991). Additionally, Bloomer et al. (2005) reported that anaerobic exercise (i.e., resistance training and sprinting) also improves AOC by concomitant exposure to exercise mediated increased ROS, inflammation, and ischemia (Bloomer et al., 2005). However, little information is available on how anaerobic and aerobic exercise training differentially affect ROS and antioxidant levels. Furthermore, the production of OS in response to different types of exercise such as aerobic and anaerobic exercise has not been investigated in human subjects. Therefore, the purpose of this study was to investigate the resting and postexercise redox status in two distinct training groups: aerobically trained athletes (ET), anaerobically trained athletes (RT), and untrained individuals (UT). We hypothesized that ET would have lower resting OS and higher AOC levels compared to RT. Our secondary hypothesis was that ET would have lower OS and higher AOC levels in response to graded exercise test (GXT) compared to RT. Our tertiary hypothesis was that ET and RT would have greater redox status at rest and post-GXT compared to UT. This study will help to clarify the specific types of exercise training-dependent adaptations in redox status in humans.
MATERIALS AND METHODS
Subjects and experimental design
We recruited healthy young males, 18–35 yr of age to participate in this study. Subjects were limited to men because estrogen has been found to decrease markers of OS during both the follicular and luteal phases of the menstrual cycle (Massafra et al., 2000). The study consisted of three distinct groups; (a) ET: competitive endurance athletes (i.e., distance runners, cyclists, triathletes), (b) RT: resistance trained athletes, (i.e., football players, power lifters), and (c) UT: untrained individuals, meaning they were not engaging in regular endurance or resistance training. ET were excluded from the study with a peak oxygen consumption below 60 mL/kg/min. RT and UT were recruited based on their training status. Based on previous studies, we recruited 20 subjects per group (Table 1). Smokers and persons with cardiovascular and other contraindications to exercise were excluded from the study (Whaley-Connell et al., 2006). Subjects made 2 visits to the exercise physiology lab in the excise science center and human performance lab in Dong-Eui University. On the first visit subjects received a complete explanation of the study protocol and completed the medical history questionnaire and consent form approved by Dong-Eui University Institutional Review Board. Upon inclusion in the study, subjects completed a treadmill peak oxygen consumption (VO2peak) test (GXT) with a blood draw taken before and after the test in the second visit to the laboratory.
Exercise test
A GXT on a treadmill was performed to volitional fatigue. Expired respiratory gases were collected through open circuit spirometry during the GXT for the determination of aerobic power or VO2peak. Expired gases were analyzed with the aid of an automated metabolic measurement cart for the determination of oxygen consumption (Max II, Physiodyne, Aei Technologies Inc., Pittsburgh, PA, USA). Subjects warmed up on the treadmill at a self-selected walk/jog pace at 3% elevation for 3 min. The treadmill grade was then lowered and the subjects ran at a moderately fast self-selected pace. The treadmill elevation was increased 1.5% every minute until the subject indicated to stop the test or any contraindications arose as per American College of Sports Medicine (ACSM) guidelines (Whaley-Connell et al., 2006). Upon termination of the test the treadmill was slowed and the elevation decreased. Heart rate (HR) and rhythm were monitored continuously for arrhythmias and ischemia during the GXT via electrocardiography. Following the GXT, the subjects were monitored for adverse symptoms until HR and blood pressure (BP) returned to normal levels as suggested by the ACSM (HR<100 beats/min; BP =pretest values) to ensure subject safety upon leaving the testing site.
Body composition and anthropometry
Participants’ standing height and weight (lightly dressed and barefoot) were measured using a calibrated scale. Thereafter, body mass index (BMI) was calculated for each subject. BMI>30 kg/m2 resulted in exclusion from the control group. Athletes with a BMI>30 kg/m2 were further evaluated by skinfold testing, and included in the study with a % body fat <20%. Skinfold thickness was measured at 3 sites (chest, abdomen, and thigh) using a Lange caliper, and body fat percentage was calculated using the formula suggested by Siri (1993).
Blood sampling
Subjects came into the lab following an 8–12 hr fast before pretest blood draws (Bloomer et al., 2005). In addition, subjects did not perform exercise training or participate in sporting events within two days before the taking of resting blood samples. A blood draw was taken by venipuncture 30 min before the GXT and 15 to 20 min following exercise. Each blood draw consisted of ~5 to 10 mL (1–2 teaspoons) of blood. Blood was centrifuged and separated for preparation of serum and plasma and was frozen at −80°C for later analysis. Samples were assayed for markers of ROS activity, malondialdehyde (MDA), protein carbonyls (PC), AOC, and total antioxidant capacity (TAC) by assay kit and spectrophotometry (Bloomer et al., 2005).
Dietary requirements
For this study, restriction of antioxidant and vitamin supplementation was very important because these supplements affect AOC. Subjects did not take vitamin supplements one week prior to testing. Subjects were asked to refrain from consuming the foods which contain high antioxidant and/or beverages for three days prior to testing (Manach et al., 2004). Otherwise, participants were asked to follow their normal diet. Subjects were asked to keep a 3-day food intake journal prior to the GXT to ensure compliance.
Statistical analysis
Significant changes over time (pre, post) and between the three different groups were assessed using a repeated measure analysis of variance. Statistical significance was established at P<0.05. Differences were further evaluated using Tukey test for multiple comparison.
RESULTS
Subject characteristics
VO2peak values for ET, RT, and UT were 68.2±3.4, 44.5±3.25, and 42.6±3.05 mL/kg/min, P<0.05, respectively. ET had the highest VO2peak values and, there were no significant differences in VO2peak between UT and RT (Table 1).
OS markers
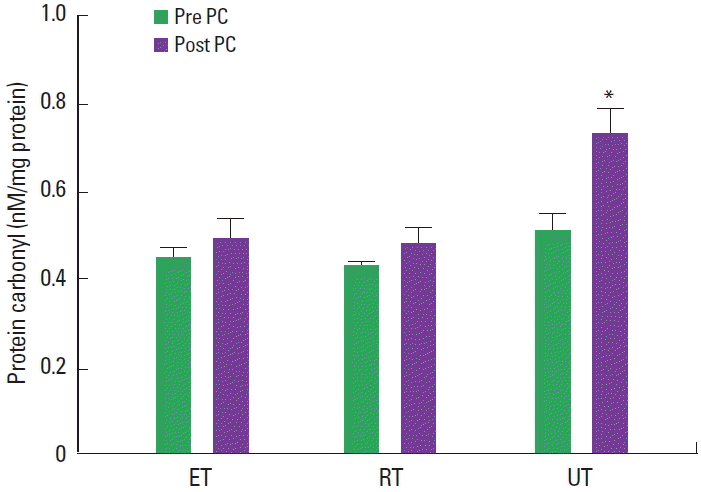
There were no significant differences in resting MDA values between ET, RT, and UT (0.79±0.8, 0.78±0.1, and 0.83±0.2 μmol/L, respectively) (Fig. 1). Resting PC levels were not different in these distinct groups. However, both MDA and PC levels significantly increased following GXT in UT but not in ET and RT (MDA vs ET, 0.79±0.8 μmol/L vs 0.78±1 μmol/L; MDA vs RT, 0.78±0.1 μmol/L vs 0.75±0.2 μmol/L; MDA vs UT, 0.83±0.2 μmol/L vs 0.98±1 μmol/L; PC vs ET, 0.45±0.3 nM/mg protein vs 0.50±0.2 nM/mg protein; PC vs RT, 0.43±0.2 nM/mg protein vs 0.48±0.3 nM/mg protein; PC vs UT, 0.51±0.4 nM/mg protein vs 0.72±0.5 nM/mg protein, P<0.05) (Fig. 2).
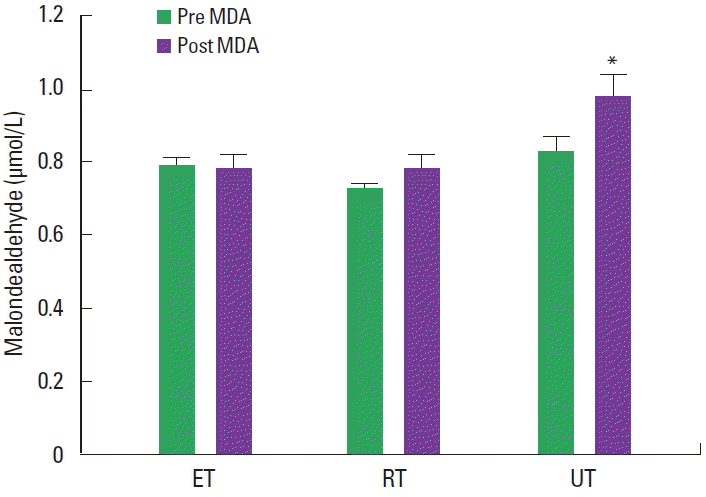
Malondealdehyde (MDA) levels at rest and post graded exercise test in groups. ET, aerobically trained athletes; RT, anaerobically trained athletes; UT, untrained individuals. Values are presented as mean± standard error. *P<0.05, significantly different from the pre MDA.
AOC marker
Resting TAC levels were not different in ET, RT, and UT. However, TAC levels significantly decreased following the GXT in all groups (TAC vs ET, 3.21±0.12 mM vs 1.24±0.22 mM; TAC vs RT: 3.38±0.13 mM vs 1.31±0.21 mM; TAC vs UT, 3.41±0.16 mM vs 0.81±0.24 mM, P<0.05, respectively). Additionally, UT showed significantly lower post TAC levels compared to ET and RT (1.24±0.22 mM vs 1.31±0.21 mM vs 0.81±0.24 mM, P<0.05, respectively) (Fig. 3).

Plasma total antioxidant capacity levels (TACs) at rest and post graded exercise test in groups. ET, aerobically trained athletes; RT, anaerobically trained athletes; UT, untrained individuals. Values are presented as mean± standard error. *P<0.05, significantly different from the pre TAC. †P< 0.05, significantly different from ET and RT.
DISCUSSION
The purpose of this study was to examine the chronic effects of different types of exercise training on OS and AOC levels in three distinct training groups. VO2peak for the ET group was in the superior category and was significantly higher than RT and UT groups. The RT and UT groups had similar VO2peak, body mass, and BMI. Many studies have shown that an acute bout of high intensity exercise increases OS production (Alessio et al., 1997; Ji, 1999; Powers and Lennon, 1999). However, chronic aerobic exercise training has been shown to attenuate OS due to increased endogenous AOC and/or enhanced function of the electron transport system, which prevents electron leakage (Alessio et al., 2000; Ji, 1995; Kim et al., 1996). There are only few studies comparing the resting endogenous MDA values between aerobically trained athletes and untrained individuals, and these studies suggest that aerobic exercise training leads to a decrease in markers of resting OS. Niess et al. (1996) showed that trained long-distance runners had lower resting MDA values compared with untrained controls. Furthermore, Knez et al. (2007) showed that ironman athletes had significantly lower resting MDA levels compared with age-matched sedentary subjects. However, these previous studies never compared resting OS levels between anaerobically trained and untrained individuals. The present work is the first study to compare redox status in aerobically and anaerobically trained athletes, further compared to untrained individuals. Additionally, there are few studies examining the chronic effects of resistance training on resting OS levels (Alessio et al., 2000; Lee et al., 2002; Zembron-Lacny et al., 2008). Furthermore, only one study has compared resting MDA levels between anaerobically trained athletes and sedentary controls, with results suggesting that anaerobic athletes maintain significantly greater resting OS levels than controls (Marzatico et al., 1997). In the current study, resting MDA and PC levels were not significantly different between the three distinct groups. However, the UT group showed significantly increased MDA and PC levels following GXT, which was not seen in the ET and RT groups. In addition, the ET and RT groups did not show any differences in post-GXT MDA and PC levels. Such findings differ from previous studies comparing plasma MDA levels in response to a treadmill GXT in cyclists and untrained individuals (Fatouros et al., 2004; Szcześniak et al., 1998). Our findings, which showed no changes in OS levels in response to GXT in the ET and UT groups, may be due to the adaptations from both aerobic and anaerobic exercise training which could result in suppressed OS production or greater antioxidant defensive capacity. This potential explanation was confirmed, at least in part, by the postexercise TAC levels, which showed that TAC levels in response to GXT were lower in the UT as compared to the ET and RT groups. In other words, endogenous AOC is lower in UT compared to both ET and RT, and the GXT mediated excessive OS production may not be well scavenged in UT compared to both ET and RT.
Interestingly, resting TAC levels showed no differences between groups. In contrast, Marzatico et al. (1997) showed that endurance runners and sprinters had significantly higher levels of AOC compared to untrained controls. Subject characteristics in the Marzatico study were similar to our subjects: untrained, sprinters, and long distance runners. However, subjects in the Marzatico study had taken antioxidant supplements habitually and they did not have a cleansing period from supplementation prior to the experiments. Even though they did not report details about supplementation, their long-distance runners took 20% higher doses of antioxidant supplements than the other two groups (Marzatico et al., 1997). Therefore, the findings in the present study are more able to represent chronic aerobic exercise training mediated true endogenous OS and AOC.
There were several limitations that could have affected the outcomes of this study. First, the GXT protocol intensity and/or duration may have been insufficient to stimulate lipid peroxidation in our trained groups. Second, MDA and PC may not be the most accurate markers of OS. Third, since we collected blood samples within 30 min after GXT, exercise blood sampling timing might have been too short to see significant expression of lipid oxidation in the blood. Fourth, TAC is too general a marker of AOC to see the differences in these groups, which warrants further investigations.
We have approached two conclusions based on the current data. First, there is no difference in resting and postexercise OS and AOC between endurance and resistance trained athletes. However, these two trained groups showed significantly higher redox balance, OS and AOC, compared to UT. Although the resistance trained and endurance trained athletes demonstrated distinct aerobic power and body composition characteristics, there were no visible OS or AOC effects between groups at rest or post exercise. Thus, both aerobic and anaerobic exercise training are beneficial to improve redox balance in humans, and any type of exercise training will be beneficial for improving redox balance against potential risk factors of excessive ROS mediated diseases
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.