Frequency-effect of playing screen golf on body composition and golf performance in middle-aged men
Article information
Abstract
There are many studies showing that physical training improves body composition including bone mineral density (BMD) in almost all subjects. However, the frequency-dependent effect of playing golf on body composition is still not clearly comprehended. Moreover, the effect of screen golf in relations with exercise-frequency on body composition and golf performance has not been documented. Forty year old men participated and were classified into 4 groups: Control group (n= 10), BMD1 group (n= 10) played screen golf less than 1 day per a week, BMD2–3 group (n= 10) played screen golf 2–3 days per a week, and BMD5 group (n= 10) played screen golf 5 days per week. Dual-energy X-ray absorptiometry (DXA) was performed on 30 male recreational golfers and 10 sedentary individuals. The data gained through DXA were fat mass, lean mass, regional (head, rib, arm, leg, pelvis, spine and trunk) BMD level, and total BMD level summed by regional scores. The club speeds were measured using the Golfzon Vision machine and the handicap points were measured using a simple questionnaire. The present results suggest that the long-frequency of playing screen golf does not improve bone mineral density, lean mass, and handicap point yet improves fat mass and club speed in the middle-aged men.
INTRODUCTION
In the middle-aged men, a healthful body composition begins to change negatively and a great amount of bone content begins to vanish by the aging process. Especially, a lot of people have interested in the bone health since they have known it was difficult to recover when bone diseases such as osteoporosis caused. The pathological condition associated with an increment of bone mass loss is defined as osteopenia, and it is caused by increased bone reabsorption. As bone mineral density (BMD) declines, the risks of bone abnormality, fragility, and fracture increase (Bemben et al., 2000). The traditional management of osteopenia comprises of pharmacologic and non-pharmacologic treatments (Lin and Lane, 2008).
Physical activity, one of the non-pharmacologic approaches, has sometimes been overlooked. The benefits of physical activity as a preventative aspect of osteopenic status are high, particularly for patients who cannot comply with medication regimens or training for weighting-lifting (Lin and Lane, 2008). The skeleton is a metabolically complex tissue that responds to a variety of stimuli including physical activity or training. Mechanical forces and loading induce changes in metabolic activity of bone and subsequently alterations in skeletal size and shape (Chen et al., 2010). The mechanical environment of the bones affects their morphology along with biochemical and cellular activities (Biewener, 1990). It is well known that physical activity or weighting-lifting could prevent and treat metabolic bone diseases (Bemben et al., 2000).
The past researches reported that the mechanical activity of the bones was closely associated with the functional status of the bone remodeling. Frost (1987) cited this linkage in proposing the mechanostat, a useful framework for considering the physical activity’s effect on bones. This model proposes a classic feedback relationship between the mechanical use and the bone strength: such that the mechanical stimulation incites the accumulation and preservation of the bone mass, while reduction in the bone loss. During a stable state of the mechanical activity, the bone balance is maintained. Based on such proposal, many researchers have reported that immobilization induces demineralization (Issekutz et al., 1966; Mack et al., 1967; Rambaut and Johnston, 1979), whereas, weighting-lifting stimulates bone accumulation (Lin and Lane, 2008; Nelson et al., 1994; Villareal et al., 2003).
Although not conclusive, the extent of the evidence for weight-bearing activities as a tool for prevention and treatment of low BMD is growing (Walters et al., 2012). Especially, the National Osteoporosis Foundation suggested which modality of weight bearing activity best develops BMD and what degree of external loading is required to maintain or promote bone health. In other words, the National Osteoporosis Foundation (2010) recommends high-impact, weight-bearing activities, such as aerobic dancing, basketball, dancing, field hockey, gymnastics, hiking, jogging or running, jumping rope, lacrosse, racquet sports, soccer, stair climbing, tennis, and volleyball, as the best exercises for keeping bones strong. A lot of physical activity types, above mentioned, were related to muscle-strengthening activities and to high-impact activities for the enhancement of BMD (Walters et al., 2012).
Although golf was not mentioned in the above recommended activity types for keeping bone health and changing body composition, golf has been reported as one of the exercise types as a strengthening activity and a high-impact on the human body (Dorado et al., 2002). Hosea and Gatt (1996) estimated the forces on the lumbar spine during golf swings. Kinetic data of subjects wearing reflective markers over the thoracic #5, thoracic #10, lumbar #1, and lumbar #3 spinous processes, in addition to the wrists, elbows, shoulders, hips, knees, ankles, and 15th metatarsal heads, was captured using 4 synchronized video cameras with high-speed shutters. Myoelectric data was collected using surface electrodes on the rectus abdominis, external oblique, and paraspinal muscles at the level of lumbar #3. In their result, the compression loads of over 8 times a person’s body weight, or about 6,100 N in amateurs and about 7,584 N in professionals, were found to be produced during a golf swing. Horton et al. (2001) also reported that a golf swing is a very complex movement which involves a lots of trunk rotation and powerful muscular contractions.
As mentioned above, golf seems to be a good exercise type to keep or maintain healthy body composition including bone mass, and provides a considerable amount of compressive forces on human bone structure. However, important questions include the frequency of exercise needed to develop or sustain a healthy body composition and bone health, and whether golf is really helpful for middle-aged men prone to osteopenia or osteoporosis. Therefore, the purpose of this study was to investigate the frequency-effect of playing golf on the BMD level in the middle-aged men. In order to obtain the results only from consistent golf swings and exclude the effects of walking the field and so on, participants using screen golf were selected.
MATERIALS AND METHODS
Subjects
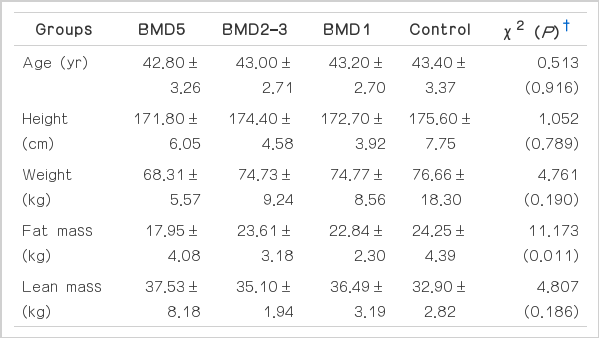
The forty men (average 43.10±2.92 years) participated in this study. The experimental design and the assessment were carried out by the Faculty of Physical Activity Design in Hanseo University after obtaining the agreement by the Seoul Song-Do Hospital Ethical Committee. The experiment was conducted from October 10 to November 2, 2013. The risks and benefits of this study were explained to all subjects, and written informed consents were subsequently obtained. The subjects were men residing around the community of screen golf facility. The average duration of playing screen golf was 5.08±3.50 yr. The subjects were classified according to the golf frequency : if one had taken part in the screen golf for 5 days or more weekly, they were classified as the BMD5 group (n = 10), if one had taken part in screen golf for 2–3 days weekly, they were classified as the BMD2–3 group (n = 10), if one had taken part in screen golf for one day weekly, they were classified as the BMD1 group (n = 10), and if one had not taken part in the screen golf, they were classified as the Control group (n = 10). In this study, in order to only analyze the frequency-effect of playing screen golf, we randomly selected the middle-aged men who were not taking calcium supplement, vitamin D, or steroid, or any medication related to BMD. Exclusion criteria included ischemic heart disease, unstable angina, dysrhythmia, recent osteoporotic fracture, and under 5 METs or less for aerobic capacity. The characteristics of all subjects are shown in Table 1.
Experimental design
Following physical examination in the hospital, the screen golfers were matched according to the exercise frequency. The participants played screen golf at a facility named Golfzon Vision (Golfzon Co., Seosan, Korea). We also measured their club speed using this Golfzon Vision and the handicap points using a simple questionnaire. Within each matching, the participants were randomly assigned into either exercise or control group. Each subject visited the research center located in Hanseo University for detailed explanation of the study procedure. All of the subjects gave written informed consent. After this procedure, each subject was tested for BMD by a radiologic technician at the Seoul Song-Do Hospital.
Measurement of body composition
The body composition was measured using Dual-energy X-ray Absorptiometry (DXA) (LUNAR, USA, 1992), a technique that has been reported to be accessible, easy to use, and able to provide an accurate estimation of BMD in adults (Dorado et al., 2002; Gilsanz, 1998). Moreover, DXA has provided a non-invasive and reliable index (Kanis et al., 2001). Therefore, we used this DXA test for measuring fat mass, lean mass and BMD level in all of the participants. Before each test, a radiologic technician was calibrated and DXA scans were performed at low speed and maximal resolution. From the whole body scans, lean mass, fat mass, and regional bone mineral density were determined. From the scans, the following regions were analyzed: head (including skull and cervical vertebrae), rib, arm (including hand and forearm), leg (including upper or lower leg and foot), pelvis, spine, trunk, and total BMD level summed by regional scores.
The T-score is the relevant measurement value for classifying normal, osteopenia or osteoporosis. The BMD of a specific location is compared to the normal reference mean values of young subjects. It is a comparison of a patient’s BMD to that of a healthy thirty-year-old. If the T-score is −1.0 or higher, it is considered normal. If it is between −1.0 and −2.5, it is considered osteopenia. Finally, when the T-score is −2.5 or lower, it is considered osteoporosis, meaning the bone density is two and a half standard deviations below the mean of a thirty-year-old man or woman.
Statistical analyses
All the measured data was processed to find the means and standard deviations using the SPSS package (version 15.0; SPSS Inc., Chicago, IL, USA). Prior to the comparative measurements of BMD, the descriptive statistics were calculated for all dependent variables. Because the data for this study was not normally distributed, non-parametric tests were used to analyze the data. The Kruskal-Wallis rank test was conducted to identify the significant differences between the groups. In addition, the ranks were obtained using the Turkey test for post hoc. The significance level for all analyses was set a priori at P≤ 0.05.
RESULTS
Anthropometric indices
The characteristics of the subjects with full data in the examinations were summarized in Table 1. Although fat mass were significantly different, no differences were observed in other characteristics among groups, indicating homogeneity in anthropometric characteristics. The results in the examinations were shown as Fig. 1. The fat masses of Control, BMD1, BMD2–3, and BMD5 groups were 24.25±4.39 kg, 22.84±2.30 kg, 23.61±3.18 kg, and 17.95±4.08 kg, respectively. The lean masses of Control, BMD1, BMD2–3, and BMD5 groups were 32.90±2.82 kg, 36.49±3.19 kg, 35.10±1.94 kg, and 37.53±8.18 kg, respectively. Fig. 1 shows that the fat mass of the BMD5 group was significantly lower than the other three groups (χ2 = 11.173; P = 0.011), whereas lean mass was not significantly different in all of the groups (χ2 = 4.807; P = 0.186).
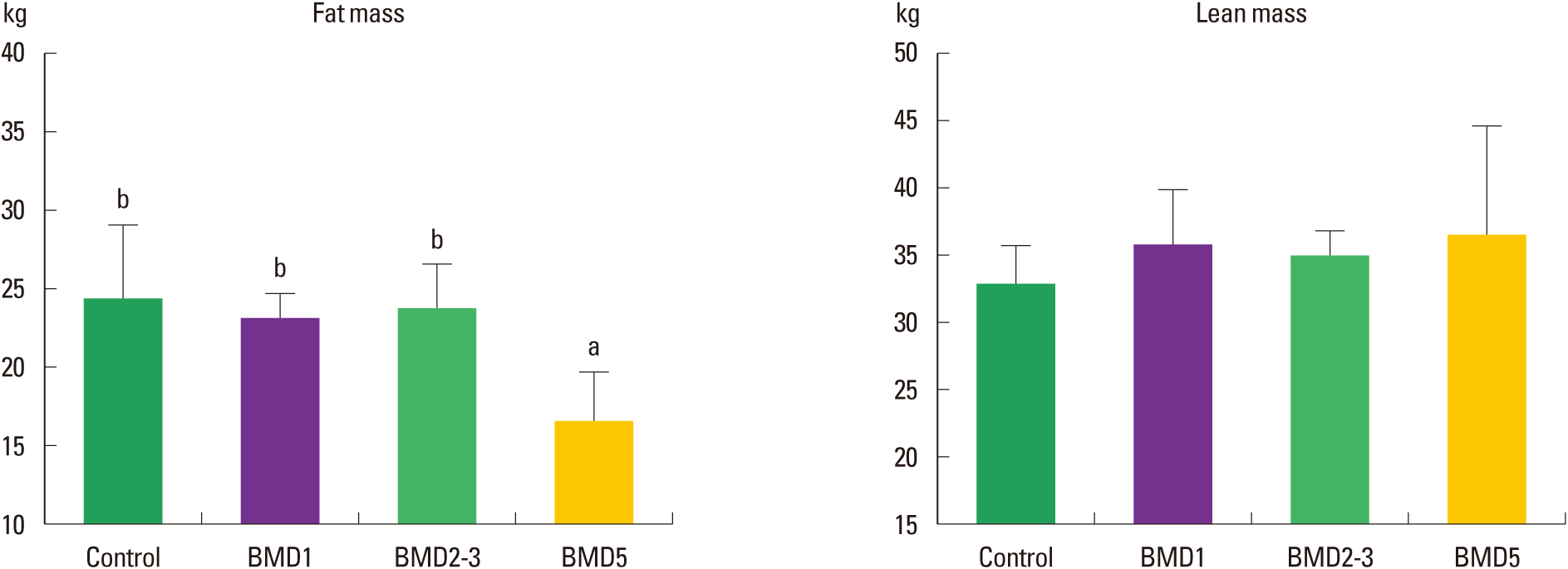
Comparative results of the fat mass and lean mass among 4 groups. In the figure, Control, BMD1, BMD2–3, and BMD5 represent control group, one day per week exercise group, 2–3 days per week exercise group, and 5 days per week exercise group, respectively. And the symbols a and b represent the post hoc results of the Turkey test using ranks.
The differences of golf handicap and club speed
The results in the examinations were shown as Fig. 2. The golf handicaps of the BMD1, BMD2–3, and BMD5 groups were 6.90±1.52 point, 7.20±1.62 point, and 6.30±2.11 point, respectively. The club speeds of the BMD1, BMD2–3, and BMD5 groups were 86.80 ±4.54 km/h, 85.40 ±6.80 km/h, and 95.60±5.91 km/h, respectively. Fig. 2 shows that the handicap points of the three groups were not significantly different (χ2 = 1.031; P = 0.597), whereas the club speed of the BMD5 group was significantly faster than the other two groups (χ2 = 9.194; P = 0.010).
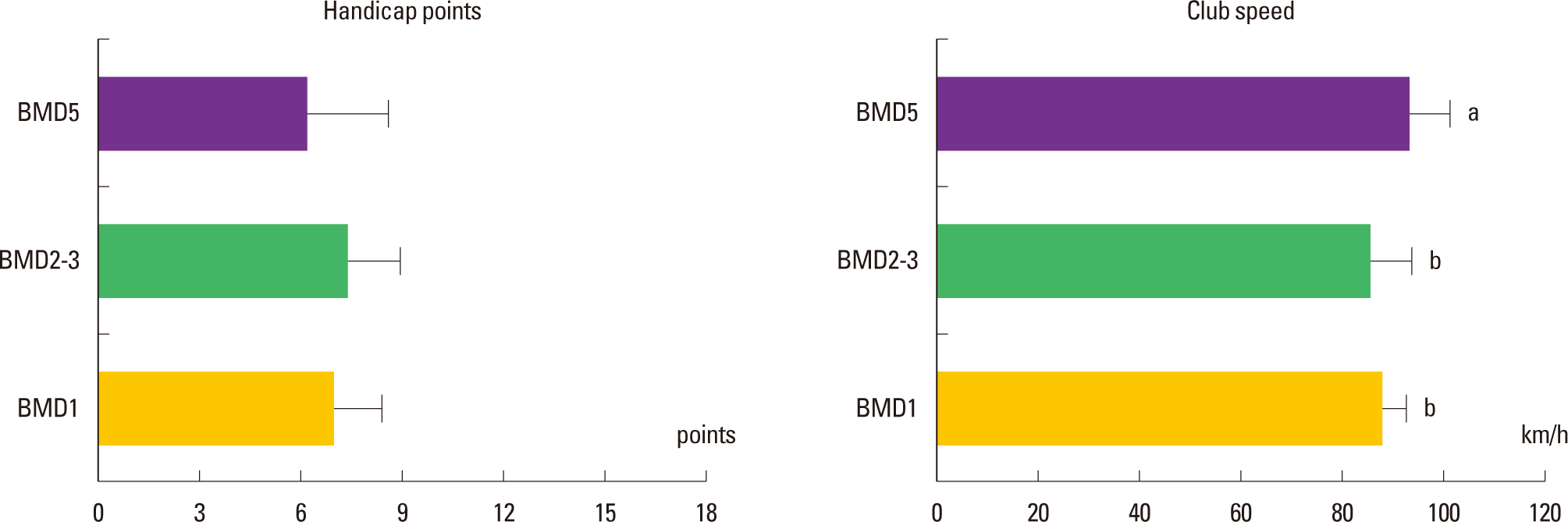
Comparative results of the handicap points and club speed among the 3 groups. In the figures, BMD1, BMD2–3, and BMD5 represent the one day per week exercise group, 2–3 days per week exercise group, and 5 days per week exercise group, respectively. And the symbols a and b represent the post hoc results of the Turkey test using ranks.
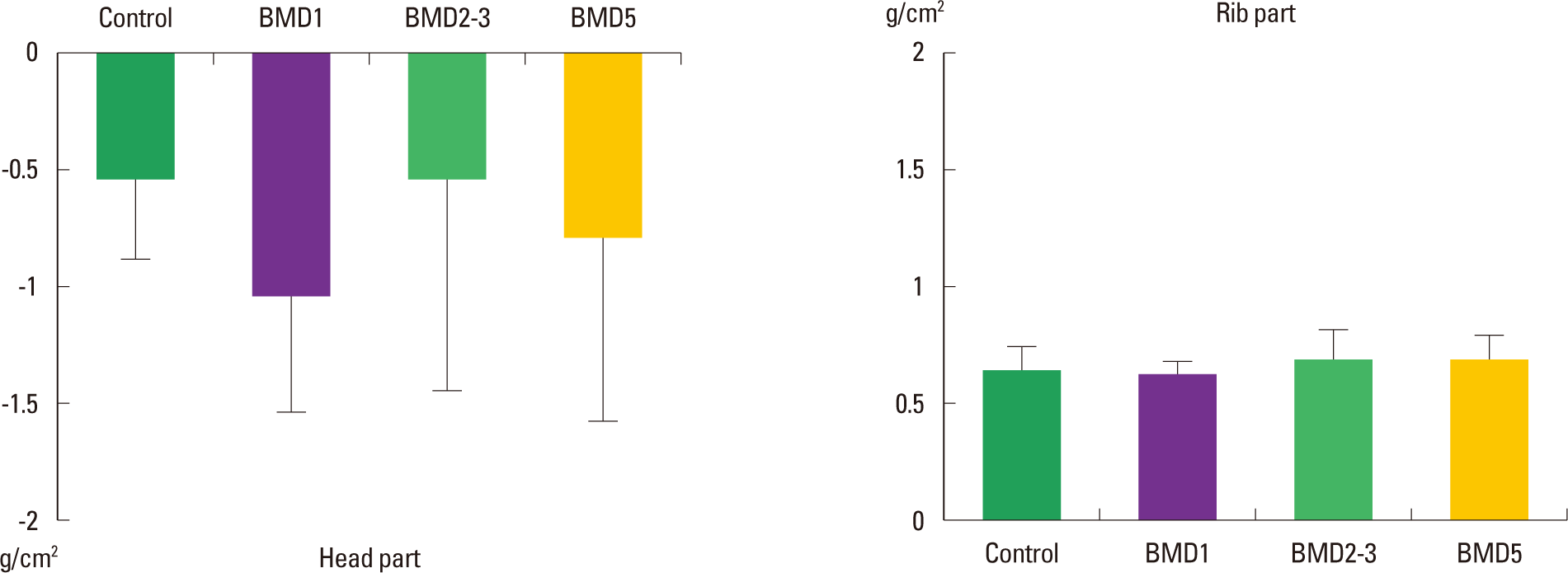
The Frequency-effects of playing screen golf on the BMD levels of Head and Rib parts
The BMD levels of the Head segment of the control, BMD1, BMD2–3, and BMD5 groups were −0.56±0.32 g/cm2, −1.10 ±0.41 g/cm2, −0.56±1.38 g/cm2, and −0.85±0.74 g/cm2, respectively. In comparison to the level of the control group, the BMD levels of the three exercised groups were not significantly different (χ2 = 5.087; P = 0.166). The BMD levels of the Rib segment of the control, BMD1, BMD2–3, and BMD5 groups were 0.64±0.06 g/cm2, 0.62±0.05 g/cm2, 0.70±0.13 g/cm2, and 0.71±0.08 g/cm2, respectively. In comparison among the groups, the BMD levels of the three exercised groups were not significantly different (χ2 = 6.723; P = 0.081) (Fig. 3).
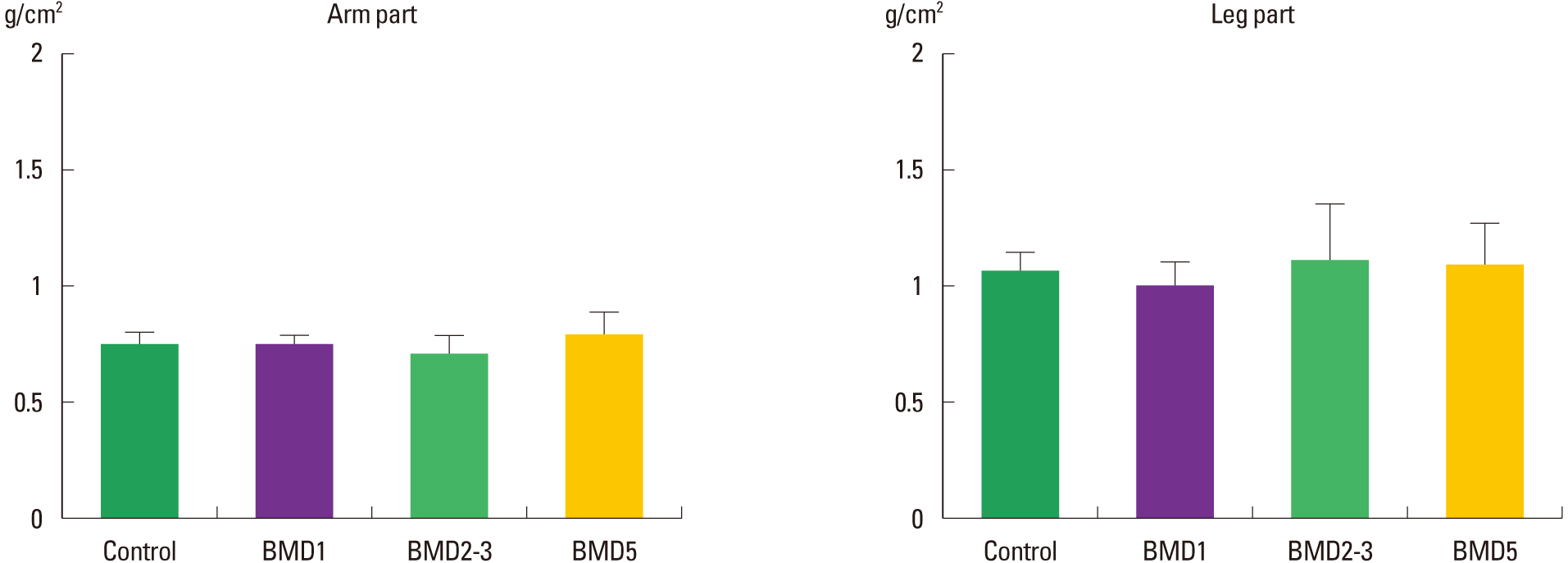
Frequency-effect of playing screen golf on BMD levels of Arm and Leg parts
The BMD levels of the Arm segment of the control, BMD1, BMD2–3, and BMD5 groups were 0.74±0.07 g/cm2, 0.75±0.05 g/cm2, 0.73±0.07 g/cm2, and 0.78±0.11 g/cm2, respectively. In comparison to the level of the control group, the BMD levels of the three exercised groups were not significantly different (χ2 = 2.073; P = 0.557). The BMD levels of the Rib segment of the control, BMD1, BMD2–3, and BMD5 groups were 1.05±0.06 g/cm2, 0.99±0.08 g/cm2, 1.17±0.23 g/cm2, and 1.12±0.16 g/cm2, respectively. In comparison among the groups, the BMD levels of the three exercised groups were not significantly different (χ2 = 6.411; P = 0.093) (Fig. 4).
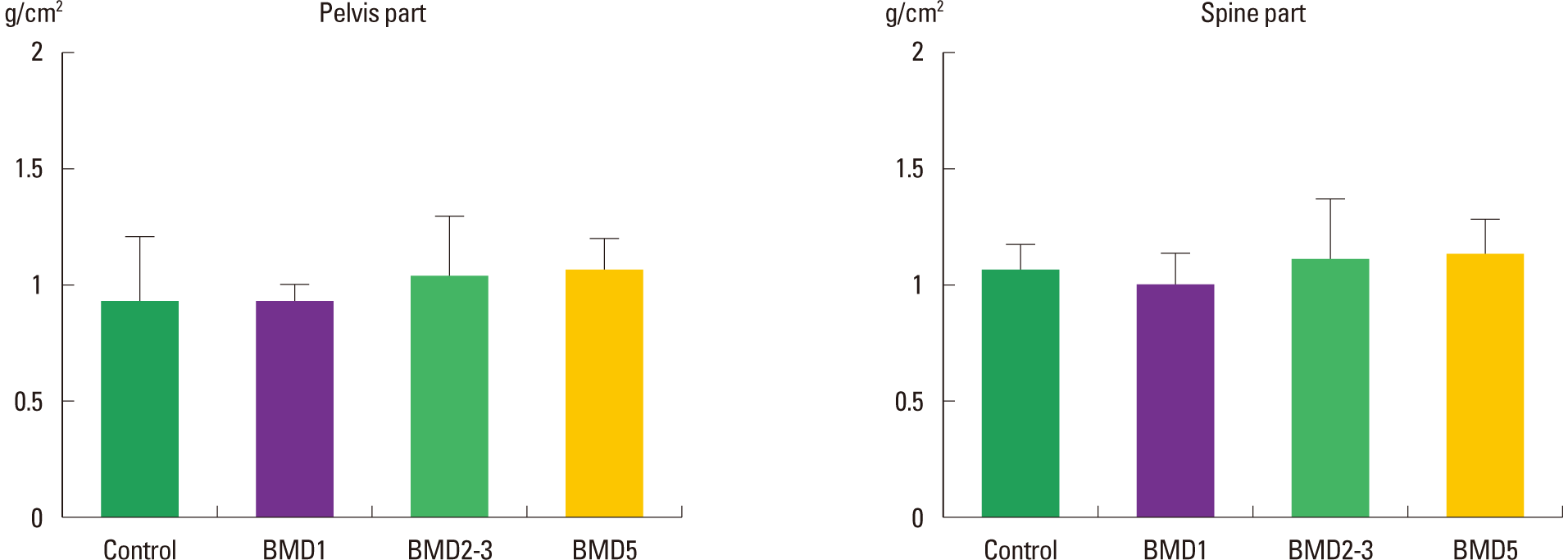
Frequency-effect of playing screen golf on BMD levels of Pelvis and Spine parts
The BMD levels of the Pelvis segment of the control, BMD1, BMD2–3, and BMD5 groups were 0.93±0.31 g/cm2, 1.01±0.08 g/cm2, 1.07±0.23 g/cm2, and 1.08±0.14 g/cm2, respectively. In comparison to the level of the control group, the BMD levels of the three exercised groups were not significantly different (χ2 = 2.862; P = 0.413). The BMD levels of the Spine segment of the control, BMD1, BMD2–3, and BMD5 groups were 1.04± 0.10 g/cm2, 0.97±0.14 g/cm2, 1.10±0.28 g/cm2, and 1.15±0.13 g/m2, respectively. In comparison among the groups, the BMD levels of the three exercised groups were not significantly different (χ2 = 5.739; P = 0.125) (Fig. 5).
Frequency-effect of playing screen golf on Trunk part and Total BMD levels
The BMD levels of the Trunk segment of the control, BMD1, BMD2–3, and BMD5 groups were 0.84±0.02 g/cm2, 0.82±0.07 g/cm2, 0.91±0.19 g/cm2, and 0.91±0.10 g/cm2, respectively. In comparison to the level of the control group, the BMD levels of the three exercised groups were not significantly different (χ2 = 4.321; P = 0.229). The BMD levels of the Total BMD levels of the control, BMD1, BMD2–3, and BMD5 groups were 1.07±0.01 g/cm2, 1.03±0.05 g/cm2, 1.09±0.13 g/cm2, and 1.13±0.12 g/cm2, respectively. In comparison among the groups, the BMD levels of the three exercised groups were not significantly different (χ2 = 5.996; P = 0.112) (Fig. 6).
DISCUSSION
Golf seems to be a good form of exercise to maintain healthy body composition including bone mass, and to provide a considerable amount of compressive force on the human bone structure. Dorado et al. (2002) reported that golf provides strengthening and a high-impact activities on a human body. Their research on golf may have included swinging and walking a long field to maintain healthy bone and body composition in the golfers. In terms of bone health and mechanical force, Hosea and Gatt (1996) reported that the golf swings provided forces on the lumbar spine of the golfers. Gluck et al. (2008) also reported that although golf may seem less physically demanding than most sports, a golf swing generates a tremendous amount of force.
There are lots of literatures available on the golf swing. It can be broken up into four basic components: backswing, forward swing, acceleration with ball strike, and follow-through (Adlington, 1996; Pink et al., 1993; Watkins et al., 1996). Gluck et al. (2008) reported that there are generally two types of swing styles: modern or classic golf swing. The modern golf swing emphasizes a large shoulder turn with a restricted hip turn. Maximizing the hip-shoulder separation angle also increases the torsional load in the spine, which serves to further stretch the viscoelastic elements. In addition, the lumbar spine is exposed to a significant compression, anterior-posterior shearing, torsion, and lateral bending forces during a golf swing. On the other hand, the classic golf swing is accomplished by raising the front heel during the backswing to increase hip turn, shortening the backswing, or a combination of the two. This reduces the magnitude of the hip-shoulder separation angle, which in turn decreases the torque on the lumbar spine (Hosea and Gatt, 1996). Due to the above reasons, although the classic swing has not been adopted as a good performance style, it has been incorporated as a treatment modality into specific rehabilitation studies (Egret et al., 2004) and as an adaptable method for the older people.
As a mentioned above, a golf swing is a high torque and high lateral bending movement, which activates a several musculoskeletal systems (Chung et al., 2014; Egret et al., 2004). In other words, a golf swing is similar to any other form of exercise that offer physical activity or a weighting-lifting movement which could prevent and treat metabolic bone diseases (Bemben et al., 2000). A number of studies have shown that exercise can increase BMD or prevent further bone loss compared with the non-exercising control groups (Kerr et al., 1996; Taaffe et al., 1996). The greatest osteogenic effect is attained when high-intensity strains are repeated regularly. However, the high strains are not the only stimulant necessary for bone formation; the frequency and number of actions also have essential roles. In other words, an exercise intervention should be performed more frequently (Rubin et al., 2006). Like many of the researches, we found that the long-frequent screen golf is related to the significant increments in the club speed as the club speed of the BMD5 group was significantly faster than the other two exercised groups.
While golf is generally less physically demanding than most sports, the golf swings generate very high loading of the lumbar spine over a short duration, due to high trunk rotation velocities and trunk muscle contraction forces (Dorado et al., 2002; Myers et al., 2008). In the related above researches, Chang et al. (2013) reported that the lumbar spine BMD was 6.7% higher in the golf players than in the control subjects. The repetitive short-duration and the high intensity spinal loading associated with the golf swings may produce the bone stain stimuli required to promote the osteoblastic activity (Burr et al., 2002; Robling et al., 2002).
Unlike previous studies, this present study contemplated by a previous research (Rubin et al., 2006), found that a long-frequent screen golf is not related with significant increments in BMD of recreational golfers. This result was similar to the results of other researches (Hatori et al., 1993; Peterson et al., 1990; Preisinger et al., 1995), the studies using low to moderate muscular endurance type activities, such as walking and aerobic dancing, demonstrated no significant effect or sometimes decreasing effect on the lumbar BMD. Many researchers also reported that moderate to high intensity exercise induced only appreciable gains in strength and variable changes to the bones (Beverly et al., 1989; Humphries et al., 2000; Nelson et al., 1994; Taaffe et al., 1996). However, Dorado et al. (2002) indicated that in the professional male golfers no increase in the forearm BMD was found compared to the non-golfing control subjects like the results of this study.
The main reason why bone mass was hardly increased in the several body parts of the subjects was because most of the golf actions are composed of medium to short distance hits. Moreover, the number of hits is much lower in the golf competitions than in any other racket sports confirmed by Dorado et al. (2002). Although playing screen golf is a high torque and weight-lifting activity, this study confirmed that the club speed increased in the BMD5 group but it was not stressful enough on the skeletal system to elicit a greater stimulus for the bone formation. In the present study, we found that the long-frequent screen golf is not related to the significant increments in BMD and lean mass, however, the fat mass showed a noticeable decrease in the long-frequent screen golfers, the BMD5 group, compared with the other three groups. Through this study, it was demonstrated that the middle-aged men could positively lose the fat mass by frequently participating for screen golf. This result, we believe, that the subjects participated in the long-frequent screen golf could consume considerable calories. From this result, it can be inferred that the decreased fat mass in the BMD5 group could contribute to the decrease in the fat mass.
In conclusion, the present results suggest that the long-frequency of playing screen golf does not improve the bone mineral density, lean mass, and handicap point. However, it improves the fat mass and club speed in the middle-aged men. Further studies are needed to determine the threshold exercise prescription that will produce significant increases in the bone mass.
Notes
CONFLICT OF INTEREST
There are no potential conflicts of interest relevant to this article.