Treadmill exercise improves motor coordination through ameliorating Purkinje cell loss in amyloid beta23-35-induced Alzheimer’s disease rats
Article information
Abstract
Alzheimer’s disease (AD) is a most common age-related neurodegenerative disease. AD is characterized by a progressive loss of neurons causing cognitive dysfunction. The cerebellum is closely associated with integration of movement, including motor coordination, control, and equilibrium. In the present study, we evaluated the effect of tread-mill exercise on the survival of Purkinje neurons in relation with reactive astrocyte in the cerebellum using Aβ25–35–induced AD rats. AD was induced by a bilateral intracerebroventricular (ICV) injection of Aβ25–35. The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 4 weeks, starting 2 days after Aβ25–35 injection. In the present results, ICV injection of Aβ25–35 deteriorated motor coordination and balance. The number of calbindin-positive cells in the cerebellar vermis was decreased and glial fibrillary acidic protein (GFAP) expression in the cerebellar vermis was increased in the Aβ25–35-induced AD rats. Treadmill exercise improved motor coordination and balance. Treadmill exercise increased the number of Purkinje neurons and suppressed GFAP expression in the cerebellar vermis. The present study demonstrated that treadmill exercises alleviated dysfunction of motor coordination and balance by reduction of Purkinje cell loss through suppressing reactive astrocytes in the cerebellum of AD rats. The present study provides the possibility that treadmill exercise might be an important therapeutic strategy for the symptom improvement of AD patients.
INTRODUCTION
Alzheimer’s disease (AD) is a most common age-related neuro-degenerative disease, and this disease characterized by a progressive loss of neurons causing cognitive dysfunction (Cahn-Weiner et al., 2002). Accumulation of amyloid beta (Aβ) plaques in the various brain regions has been suggested as the etiology of PD (LaFerla and Oddo, 2005; Reddy and Beal, 2008).
Cerebellum is closely associated with integration of movement, including motor coordination, control, and equilibrium. Purkinje neurons in the cerebellum are main types of neurons in the cerebellum, and these neurons are associated with motor control, movement learning, and sensory processing (Barski et al., 2003). Purkinje neurons are implicated in the cerebellar circuit (Abrams and Zhang, 2011).Motor dysfunction observed in the AD rats is caused by damage and death of Purkinje cells in the cerebellum (Jiang et al., 2013; Kozuki et al., 2011). In the AD disease, Purkinje cell bodies are lost and the density of dendritic arborization is significantly decreased (Mavroudis et al., 2013).
Reactive astrogliosis is a key component of the cellular response to central nervous system injury. Reactive astrocytes induce toxic edema, inflammation, release of cytotoxins, and glial scar formation that inhibit axonal regeneration and neuronal survival that exacerbate Alzheimer disease (Kamphuis et al., 2014; Myer et al., 2006). Reactive astrocyte is very sensitive markers of neuronal damage in the brain (Devinsky et al., 2013). Steel et al. (2012) suggested that reactivate astrocytes in AD increase neurofibrillary tangles in the brain. Astrocyte morphology can be assessed by glial fibrillary acidic protein (GFAP) immunostaining.
Exercise improves cognitive function and ameliorates motor dysfunction (Heo et al., 2014; Kim et al., 2011). Exercise may attenuate neurological impairments following various brain injuries such as cerebral ischemia, hemorrhage, and Parkinson’s disease (McDonnell et al., 2013; Sung et al., 2012). However, the effect of exercise on Purkinje neurons in relation with reactive astrocytes in AD has not documented. In the present study, we evaluated the effect of treadmill exercise on the survival of Purkinje neurons in relation with reactive astrocyte in the cerebellum using Aβ25–35–induced AD rats.
MATERIALS AND METHODS
Animals and treatments
The experimental procedures were conducted in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Male Sprague-Dawley rats, weighing 220±10 g (7 weeks old), were used in this experiment. Each animal was housed under controlled temperature (20± 2°C) and lighting (07:00 h–19:00 h) conditions with food and water made available ad libitum. The animals randomly were divided into 4 groups (n=10 in each group): the control group, the control and treadmill exercise group, the Aβ25–35-injection group, and the Aβ25–35-injection and treadmill exercise group.
Intracerebroventricular (ICV) administration of Aβ25–35
Aβ25–35 (Sigma Chemical Co., St. Louis, MO, USA) was dissolved in sterile double-distilled water at a concentration of 1 μg/ μL, and incubated at 37°C for 4 days for aggregation, and then stored at −20°C. The animals were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France) and placed in a stereotaxic frame. Burr holes were drilled in the skull on both the sides over the lateral ventricles using the following coordinates: 0.8 mm posterior to bregma, 1.5 mm lateral to sagittal suture, 3.6 mm beneath the surface of brain. Through a hole drilled in the skull, a 26-gauge needle inserted manually into each lateral ventricle. The lesioned groups received a bilateral ICV injection of Aβ25–35 (5 μL in saline), according to the previously described method (Tohda et al., 2003). The animals in the control group underwent the same surgical procedures, but same volume of saline was injected instead of Aβ25–35.
Treadmill exercise protocol
The rats in the exercise groups were made to run on the treadmill 30 min once a day, five times a week during 4 weeks, starting 2 days after Aβ25–35 injection. The workload of the exercise consisted of running at a speed of 3 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, with 0% grade of inclination. The animals in the control group and in the Aβ25–35-injection group were left without running for the same duration.
Rota-rod test
Four weeks after conducting treadmill exercise, we performed rota-rod (Harvard Apparatus, Holliston, MA, USA) test to measure motor coordination and balance. Each rat was placed in a separate compartment on the rotating rod (diameter, 4 cm). The rat was placed on top of the rotating rod (4 rpm) facing away from the experimenter, in the orientation opposite to that of the rod movement so that forward locomotion was necessary in order to avoid a fall. During the pre-trials, each rat was allowed to familiarize itself with the apparatus as the beam rotated at a constant speed of 4 rpm. Following the pre-trials, the rat was exposed to two test trials in which the rotation accelerated from 4 to 20 rpm. Latency until fall was automatically recorded by magnetic trip plate. To eliminate stress and fatigue, a maximum cut off latency was limited as 180 sec.
Tissue preparation
The animals were sacrificed after rota-rod test. The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). Then, they were transcardially perfused with 50 mM phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were dissected and post fixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Sagittal sections of 40 μm thickness from cerebellum were made using a freezing microtome (Leica, Nussloch, Germany).
Calbindin D-28k-positive immunohistochemistry
Immunohistochemistry for the calbindin-positive cells in the cerebellar vermis was performed. The sections were incubated in 50 mM PBS for 5 min and then washed three times in the same buffer. Free-floating sections were first incubated in 1% H2O2 for 30 min. Next, the sections were incubated in blocking solution (1% BSA and 10% horse serum in 50 mM PBS) for 2 h at room temperature. Then, the sections were incubated overnight with anti-calbindin-D-28K antibody (1:500; Sigma Chemical Co., St. Louis, MO, USA). Biotinylated anti-mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) was incubated, and then the sections were incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. For staining, the sections were incubated in a solution consisting of 0.02% 3,3-diaminobenzidine (DAB; Sigma Chemical Co.) and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for approximately 5 min, after which they were washed with PBS and mounted onto gelatin-coated slides. Cover slips were mounted using Permount® (Fisher Scientific, Fair Lawn, NJ, USA). The number of Purkinje-positive cells was quantified in a field with dimensions of 1,000 μm×400 μm in the regions of the cerebellar vermis using Image-Pro® Plus software (Media Cybernetics, Silver Spring, MD, USA).
GFAP immunohistochemistry
Immunohistochemistry for the GFAP expression in the cerebellar vermis was performed. The sections were incubated in 50 mM PBS for 5 min and washed three times in the same buffer. Free-floating sections were first incubated in 3% H2O2 for 30 min. Next, the sections were incubated in blocking solution (1% BSA and 10% horse serum for GFAP in 50 mM PBS) for 2 h at room temperature. The sections were then incubated overnight with anti-GFAP antibody (1:500; Chemicon International, Inc., Temecula, CA, USA). The biotinylated anti-mouse secondary antibody (1:200; Vector Laboratories) was incubated, and then the sections were incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. For staining, the sections were incubated in a solution consisting of 0.02% DAB and 0.03% H2O2 in 50 mM Tris-HCl (pH 7.6) for approximately 5 min, after which they were washed with PBS and mounted onto gelatin-coated slides. Cover slips were mounted using Permount® (Fisher Scientific). The density of GFAP-positive reactive astrocytes was assessed in a quantitative fashion, according to a micro-densitometrical method based on optical density using Image-Pro® Plus software (Media Cybernetics, Bethesda, MD, USA).
Statistical analysis
Data were analyzed using the statistical software PASW (version 21.0). Results were expressed as the mean±standard error of the mean (SEM). For the comparison among the groups, one-way ANOVA and Duncan’s post-hoc test were performed with P<0.05 as the indication of statistical significance.
RESULTS
Effect of treadmill exercise on rota-rod test
The latencies of the rota-rod test are presented in Fig. 1. The latency (sec) was 102.3±7.0 sec in the control group, 105.3±7.1 sec in the control and exercise group, 59.0±3.6 sec in the Aβ25–35-injection group, 83.5±4.0 sec in the Aβ25–35-injection and exercise group. Injection of Aβ25–35 deteriorated motor coordination and balance (P<0.05), whereas treadmill exercise improved motor coordination and balance in the AD rats (P<0.05).
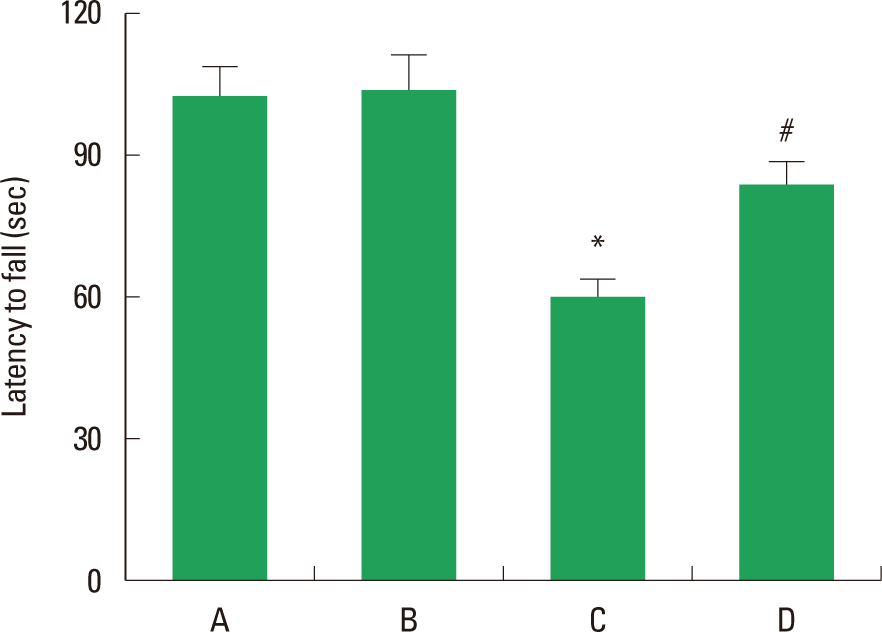
Effect of exercise on the rota-rod test. (A) Control group, (B) control and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. The data are represented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the control group. # represents P< 0.05 compared to Aβ25–35-injection group.
Effect of treadmill exercise on the number of Purkinje neurons in the cerebellar vermis
The number of calbindin-positive cells in the anterior region of cerebellar vermis was 52.68±1.86 in the control group, 51.79± 1.77 in the control and exercise group, 40.37±1.61 in the Aβ25–35-injection group, and 48.16±1.80 in the Aβ25–35-injection and exercise group (Fig. 2, left).

Effect of treadmill exercise on the calbindin-positive Purkinje neurons in the cerebellar vermis. Upper: Photomicrographs of sagittal section from cerebellular vermis. The scale bar is 100 μm. Lower: The number of calbindin-positive cell in each group. (A) Control group, (B) control and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. The data are represented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the control group. #represents P< 0.05 compared to the Aβ25–35-injection group.
The number of calbindin-positive cells in the middle region of cerebellar vermis was 52.37±2.12 in the control group, 52.11± 1.36 in the control and exercise group, 44.79±1.39 in the Aβ25–35-injection group, and 49.95±1.83 in the Aβ25–35-injection and exercise group (Fig. 2, middle).
The number of calbindin-positive cells in the posterior region of cerebellar vermis was 53.32±1.72 in the control group, 51.63± 1.64 in the control and exercise group, 39.05±1.53 in the Aβ25–35-injection group, and 45.58±2.41 in the Aβ25–35-injection and exercise group (Fig. 2, right).
Purkinje neuronal loss in the cerebellar vermis occurred by Aβ25–35-injection (P<0.05), whereas treadmill exercise alleviated AD-induced Purkinje cell loss (P<0.05).
Effect of treadmill exercise on the reactive astrocyte in the cerebellar vermis
The optical density of reactive astrocyte in the granule layer of anterior cerebellar vermis was 20.31±2.92 in the control group, 27.90±3.44 in the control and exercise group, 58.12±4.84 in the Aβ25–35-injection group, and 26.45±4.42 in the Aβ25–35-injection and exercise group. The optical density of reactive astrocyte in the molecular layer of anterior cerebellar vermis was 23.59±3.48 in the control group, 46.39±6.98 in the control and exercise group, 86.06±8.31 in the Aβ25–35-injection group, and 44.20±4.25 in the Aβ25–35-injection and exercise group (Fig. 3, left).
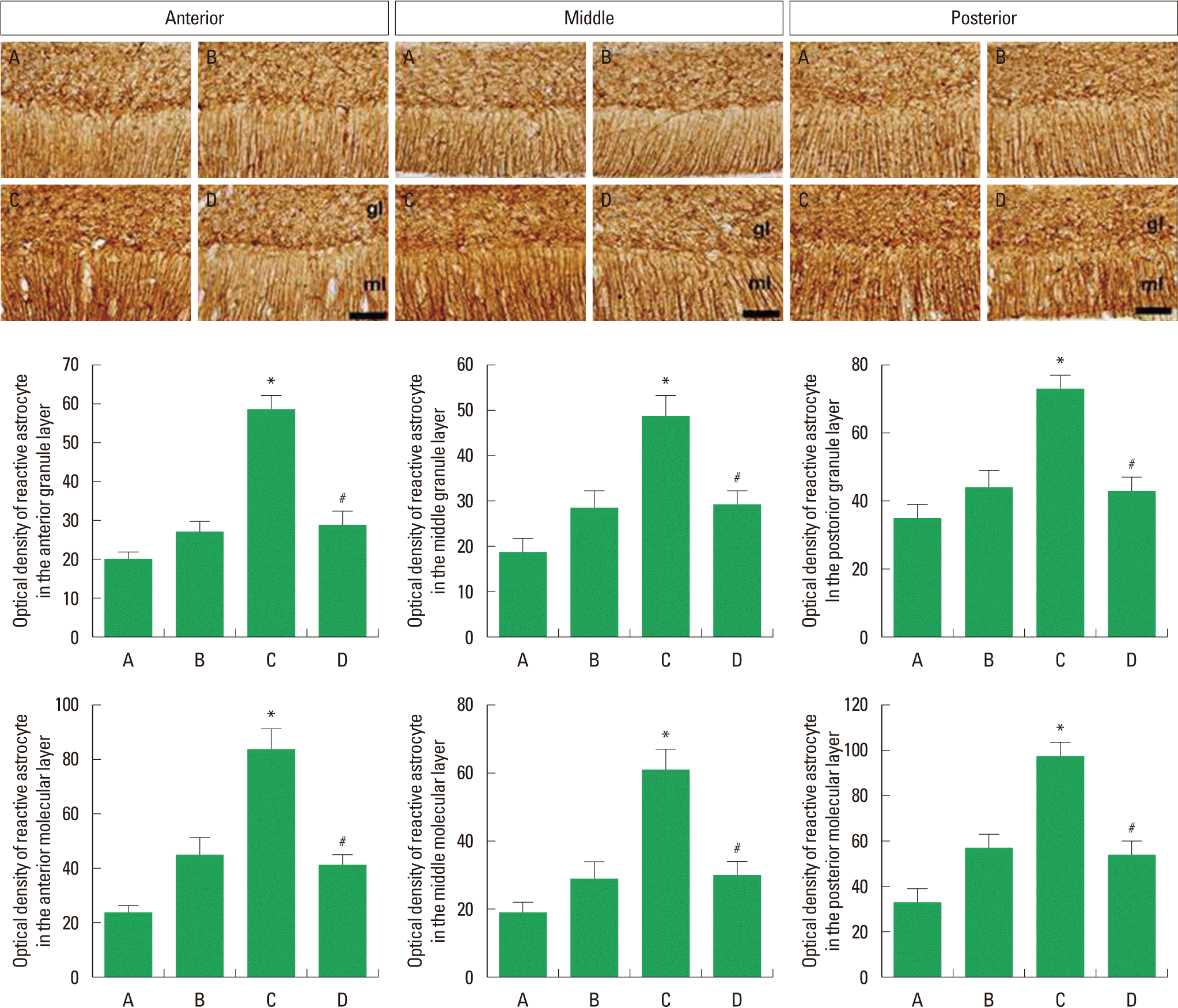
Effect of treadmill exercise on the reactive astrocyte in the cerebellar vermis. Upper: Photomicrographs of reactive astrocyte in the molecular and granule layer of cerebellar vermis. The scale bar is 100 μm. Lower: The optical density of reactive astrocytes in each group. (A) Control group, (B) control and treadmill exercise group, (C) Aβ25–35-injection group, and (D) Aβ25–35-injection and treadmill exercise group. The data are represented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the control group. #represents P< 0.05 compared to the Aβ25–35-injection group.
The optical density of reactive astrocyte in the granule layer of middle cerebellar vermis was 18.69±3.46 in the control group, 28.15±5.30 in the control and exercise group, 49.29±5.93 in the Aβ25–35-injection group, and 28.30±4.47 in the Aβ25–35-injection and exercise group. The optical density of reactive astrocyte in the molecular layer of middle cerebellar vermis was 18.97±3.65 in the control group, 29.93±6.27 in the control and exercise group, 61.96±6.45 in the Aβ25–35-injection group, and 30.93±4.47 in the Aβ25–35-injection and exercise group (Fig. 3, middle).
The optical density of reactive astrocyte in the granule layer of posterior cerebellar vermis was 33.35±4.61 in the control group, 47.20±5.16 in the control and exercise group, 71.55±4.80 in the Aβ25–35-injection group, and 46.40±5.67 in the Aβ25–35-injection and exercise group. The optical density of reactive astrocyte in the molecular layer of posterior cerebellar vermis was 33.72±6.11 in the control group, 56.37±5.74 in the control and exercise group, 93.39±7.10 in the Aβ25–35-injection group, and 50.33±7.98 in the Aβ25–35-injection and exercise group (Fig. 3, right).
Expression of reactive astrocyte in the molecular and granule layer of cerebellar vermis was increased by Aβ25–35-injection (P< 0.05), whereas treadmill exercise decreased expression of reactive astrocyte in the cerebellar vermis (P<0.05).
DISCUSSION
AD is characterized by loss of neurons and dendritic spins with degeneration of neurofibrils, mainly in the hippocampus and cerebellum (Mavroudis et al., 2010) Cerebellum modulates coordination of movement, and cerebellar dysfunction lacks coordination of voluntary movement and then causes gait disturbance (Taroni and DiDonato, 2004). Several studies suggested that AD induces motor impairments, including dysfunction of gait and balance (Inzitari et al., 2013; Kuwabara et al., 2014). In the present study, Aβ25–35-induced AD rats showed disturbance of coordination and balance in the rota-rod test. In contrast, treadmill exercise improved coordination and balance in the Aβ25–35-induced AD rats.
Previous studies suggested that coordination dysfunction by AD and traumatic brain injury is associated with Purkinje cell loss in the cerebellum (Kozuki et al., 2011; Prakash et al., 2013; Seo et al., 2010). Purkinje neurons in the cerebellum are associated with motor coordination and motor learning (Barski et al., 2003). Dysfunction of coordination is caused by a reduction in Purkinje neurons (Kim et al., 2013; Kozuki et al., 2011). In the present study, the number of calbindin-positive neurons in the anterior, middle, and posterior regions of the cerebellar vermis was decreased in the Aβ25–35-induced AD rats, whereas treadmill exercise ameliorated this loss of in the Aβ25–35-induced AD rats.
In animal model of AD, the reduction of Purkinje neurons in the cerebellum is closely associated with reactive astrocytes (Sekiguchi et al., 2003). Reactive astrocytes contribute to neurodegeneration in the AD (Verkhratsky et al., 2010). GFAP expression is associated the plaque load with the number of neurofibillary tangles (Kamphuis et al., 2012). Recently, it was reported that GFAP expressing in the brain was increased during the progress of AD (Kamphuis et al., 2014). In the present study, we demonstrated that optical density of reactive astrocytes was increased in the granule and molecular layer of cerebellar vermis in the Aβ25–35-induced AD rats, whereas treadmill exercise inhibited expression of reactive astrocytes in the Aβ25–35-induced AD rats.
Exercise is known to inhibit neuronal degeneration in the Parkinson’s disease (O’Dell et al., 2007; Petzinger et al., 2007). In the present study, treadmill exercises also alleviated dysfunction of motor coordination and balance by reducing Purkinje cell loss through suppressing reactive astrocytes in the cerebellum of AD rats. These results suggest that treadmill exercise enhanced the survival rate of Purkinje neurons via down-regulation of reactive astrocytes. Therefore, the present study provides the possibility that treadmill exercise might be an important therapeutic strategy for the symptom improvement of AD patients.
Acknowledgements
This work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2010-327-G00099).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.