AbstractPolydeoxyribonucleotide (PDRN), which is adenosine A2A receptor agonist, facilitates healing and inhibits inflammation and apoptosis. The effect of PDRN on alcoholic liver injury (ALI) was evaluated focusing on the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) signaling pathway. The mice were given daily oral administration of 50% ethanol at a dose of 4 g/kg during 8 weeks. After 4 weeks of alcohol intake, 200 μL of normal saline containing 8-mg/kg PDRN was intraperitoneally administered 3 times a week for 4 weeks. To determine whether the action of PDRN occurs through the adenosine A2A receptor, 8-mg/kg 3,7-dimethyl-1-propargylxanthine (DMPX) with PDRN was treated. The concentration of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was detected. For liver histopathological score, hematoxylin and eosin staining was conducted. Enzyme-linked immunoassay was used to measure cyclic adenosine-3′,5′-monophosphate (cAMP) concentration. PI3K and Akt expression was determined using Western blot analysis. In the results, PDRN treatment suppressed AST and ALT level in serum and liver tissue, and improved damaged liver tissue and decreased histological score. PDRN application inhibited the expression of phosphorylated PI3K/Akt signaling pathway. The increasing effect of PDRN on cAMP level ats as a mechanism for ALI treatment. Co-treatment of DMPX with PDRN did not reduce apoptosis, causing no improvement in liver function. As a result of this experiment, PDRN has the potential to be selected as a therapeutic agent for ALI.
INTRODUCTIONThe live is the main organ of metabolism. Alcohol abuse is a frequent cause of chronic liver disease, cirrhosis, hepatocellular carcinoma and is a leading cause of indication for liver transplantation (Seitz et al., 2018). Alcoholic liver injury (ALI) is an acute or chronic disease caused by excessive alcohol consumption. Causes of ALI include inflammation, apoptosis, and fibrosis (Shojaie et al., 2020). The action of apoptosis is to keep cells functioning normally by eliminating harmful or severely damaged cells (Ko et al., 2020a; Shojaie et al., 2020). However, excessive hepatocellular death has the potential to cause liver dysfunction, causing to ALI (Wang et al., 2019; Zhang et al., 2020).
The signal transduction pathway phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) regulates cell apoptosis, metabolism, proliferation, and growth (Mayer and Arteaga, 2016; Zhang et al., 2019). Increased apoptotic expression with enhancing PI3K/Akt signaling activity reduces hepatic perfusion and eventually accelerates cirrhosis (Wang et al., 2019). In addition, inhibition of the PI3K/Akt signaling pathway has been suggested to be effective in the treatment of hepatic fibrosis and apoptosis (Son et al., 2013). Thus, PI3K/Akt signaling activity is a major pathological process leading to hepatic apoptosis, which also provides a target for the treatment of alcoholic liver toxicity.
G protein-coupled receptors expressing on immune cells, A1, A2A, A2B, and A3, mediate various actions of adenosine. Polydeoxyribonucleotide (PDRN), an adenosine A2A receptor agonist, was extracted from salmon sperm, and exerts an anti-inflammatory effect by suppressing the secretion of pro-inflammatory cytokines (Ko et al., 2021). Stimulation of A2A by PDRN decreases the production of pro-inflammatory cytokines and suppresses apoptotic cell death under various disease conditions (Hwang et al., 2021; Ko et al., 2020a). PDRN facilitates healing and suppresses apoptosis, but the exact action of PDRN for alcoholic liver damage is not clarified. In this study, effect of PDRN on alcohol-induced ALI was evaluated focusing on the PI3K/Akt signaling pathway.
The concentration of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was detected. For liver histopathological score, hematoxylin and eosin (H&E) staining was conducted. Enzyme-linked immunoassay (ELISA) was used to measure cyclic adenosine-3′,5′-monophosphate (cAMP) concentration. PI3K and Akt expression was determined using Western blot analysis.
MATERIALS AND METHODSAnimalsMale ICR mice weighing 30±2 g (10 weeks old), were obtained from a commercial breeder (Orient Bio Co., Sungman, Korea). The mice were classified into four groups (n=10 in each group): control group, ALI-induced group, ALI-induced and PDRN-treated group, ALI-induced and PDRN with 3,7-dimethyl-1-propargylxanthine (DMPX)-treated group. The experimental process was approved by the Institutional Animal Care and Use Committee of Kyung Hee University (KHUASP[SE]-20-495).
Inducing ALIIntragastric administration of ethanol was continued during 8 weeks to cause a more advanced stage of ALI. As mentioned method (Buko et al., 2019), the mice were given daily oral administration of 50% ethanol at a dose of 4 g/kg during 8 weeks. After 4 weeks of alcohol intake, 200 μL of normal saline containing 8 mg/kg PDRN (Rejuvenex, PharmaResearch Products, Panyo, Korea) was intraperitoneally administered 3 times a week for 4 weeks (12 times in total). PDRN concentration was selected as the most effective according the previous studies (Ko et al., 2020a; Ko et al., 2021). To determine whether the action of PDRN occurs through the adenosine A2A receptor, 8-mg/kg DMPX (Sigma-Aldrich Co., St. Louis, MO, USA) with PDRN was treated.
Blood sampling and tissue preparationAfter 8 weeks of experiment, all mice were anesthetized by Zoletil 50 (10 mg/kg; Vibac Laboratories, Carros, France). Blood sampling was done by heart puncture and serum was obtained by centrifugation at 3,000 rpm for 20 min. Liver tissues were taken from the left lobe and then fixed in 4% paraformaldehyde, dehydrated by ethanol (70%, 80%, 90%, 95%, and 100%), incubated with xylene, and then embedded in paraffin. Paraffin-embedded liver tissues with 5-μm thickness were made using a microtome (Thermo Fisher Scientific, Waltham, MA, USA). Slides with liver tissue were dried overnight at 37°C on a hot plate.
H&E stainingH&E staining on the liver tissues was done to detect histological changes as mentioned method (Ko et al., 2020a). The slides were incubated into Mayer’s hematoxylin (DAKO, Glostrup, Denmark) for 30 sec and then washed with tap water. The slides were incubated with eosin (Sigma-Aldrich Co.) for 10 sec and washed with water. The slides were air-dried at room temperature and then dipped twice into 95% ethanol, twice into 100% ethanol, twice into a solution of 50% ethanol and 50% xylene, and twice into 100% xylene. The histological score for tissues damage was determined as mentioned method (Chen et al., 2020). Images from H&E staining slides were obtained using an Image-Pro plus computer-assisted image analysis system (Media Cybernetics Inc., Silver Spring, MD, USA) and observed using an optical microscope (Olympus, Tokyo, Japan). For histological score, <25% of cells contain fat were +1, 26% to 50% were +2, 51% to 75% were +3, >75% were +4. For calculation of inflammation and necrosis, one lesion/lobular were +1, and two or more lesions/lobular were +2.
Measurement of AST and ALT levelSerum AST and ALT level was measured by the AST and ALT activity assay kits (AM 101-K, Asan Pharm Co., Seoul, Korea) as mentioned method (Kim et al., 2021a). The Multiskan Go microplate reader (Thermo Fisher Scientific) was used with absorbance at 510 nm.
Measurement of cAMP concentrationscAMP level in the serum and liver was determined by ELISA. By manufacturer’s instructions, the cAMP assay was done by an cAMP enzyme immunoassay kit (Abcam, Cambridge, UK) as mentioned method (Ko et al., 2020a).
Western blottingWestern blot analysis was done as mentioned method (Kim et al., 2021b; Ko et al., 2020b). The liver tissues were homogenized on chilled radioimmunoprecipitation assay buffer (Cell Signaling Technology, Danvers, MS, USA) with 1 mM phenylmethylsulfonyl fluoride (Sigma-Aldrich Co.), and then centrifuged at 14,000 rpm for 30 min at 4°C. Protein contents were measured using a μ-drop reader (Thermo Fisher Scientific), and 30-μg protein was separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred to a nitrocellulose membrane. The primary and secondary antibodies used are as shown in Table 1. After the reaction, enhanced chemiluminescence detection kit (Bio-Rad, Hercules, CA, USA) was used for blot membrane. Image-Pro plus computer-assisted image analysis system (Media Cybernetics Inc.) was used for detected bands. The control group was set to 1.00.
RESULTSConcentration of AST and ALTThe concentration of AST and ALT level in ALI is demonstrated in Fig 1. The concentration of serum AST and ALT was increased by alcohol application, whereas, PDRN treatment decreased AST and ALT level. In order to understand the adenosine A2A receptor involvement of PDRN effect, the reduction effect of PDRN on AST and ALT disappeared when PDRN and DMPX were cotreated.
Histological scoreThe histological change of ALI is demonstrated in Fig. 2. Alcohol treatment caused damage to the liver parenchyma, resulting in infiltration of inflammatory cells, periportal necrosis, hepatic edema, and hepatocellular degeneration. These changes led to elevated histological score. PDRN application suppressed these changes and reduced liver histological score. In order to understand the adenosine A2A receptor involvement of PDRN effect, the suppressing effect of PDRN on liver histological score disappeared when PDRN and DMPX were cotreated.
cAMP concentrationThe concentration of cAMP in the serum and liver tissue is demonstrated in Fig. 3. PDRN application enhanced the concentration of cAMP level in the serum and liver tissue. In order to understand the adenosine A2A receptor involvement of PDRN effect, the enhancing effect of PDRN on cAMP level disappeared when PDRN and DMPX were cotreated.
PI3K/Akt signaling expressionThe effect of PDRN on PI3K/Akt signaling expression was studied by Western blot (Fig. 4). Alcohol treatment enhanced PI3K expression and phosphorylation of Akt expression in liver tissue, suggesting PI3K/Akt pathway activation. However, PDRN treatment reduced PI3K expression and phosphorylation of Akt. In order to understand the adenosine A2A receptor involvement of PDRN effect, the suppressing effect of PDRN on PI3K expression and phosphorylation of Akt disappeared when PDRN and DMPX were cotreated.
DISCUSSIONProlonged drinking causes varying degrees of liver damages and increases the risk of developing alcoholic hepatitis or more serious ALI (Kim et al., 2008). The need for the development of effective drugs to reduce the damage caused by ALI has emerged. This study evaluated the effect of PDRN against ALI.
Elevated serum AST and ALT level is well known to suggest alcoholic liver damage (Chen et al., 2020; Hyder et al., 2013). ALT is rarely found in normal serum samples, whereas it is present in cytoplasmic mitochondria and hepatocytes (Hyder et al., 2013). In the current result, oral administration of alcohol with 8 weeks significantly increased serums AST and ALT levels, showing ALI. This study demonstrated that chronic alcohol abuse disrupted liver parenchyma, infiltration of inflammatory cells, periportal necrosis, hepatic edema, and hepatocellular degeneration. These changes caused an increase in histological score. This conclusion is similar to previous studies showing alcohol-induced histological damage to the liver (Chen et al., 2021; Luo et al., 2021).
Apoptotic cell death pathway has been implicated in the pathogenesis of ALI to important extent (Shojaie et al., 2020). Liver damage is induced by excessive apoptosis, and ALI treatment also causes mitigation of apoptosis. PI3K/Akt signaling pathway causes cell activation, which is associated with other pathways that regulate biological processes related to apoptotic cell death in liver (Chen et al., 2018; Sun et al., 2018). In particular, Akt is a survival kinase and is targeted and regulated by PI3K (Sun et al., 2018). Excessive alcohol intake enhanced phosphorylation of PI3K/Akt signaling in rodent (McCullough et al., 2016). In the present results, ALI activated Akt by enhancing PI3K expression.
cAMP reduces Akt kinase activity and inhibits Akt phosphorylation, and then modulates apoptosis (Jiang et al., 2021; Wahlang et al., 2018). Adenosine A2A receptor stimulation increases cAMP level and this increased cAMP level inhibits apoptotic cell death by secreting anti-apoptosis proteins, which in turn play a major role in the regulation of apoptosis (Ahsan and Mehal, 2014; Ko et al., 2020a). In the current study, PDRN treatment suppressed AST and ALT levels in serum and liver tissue, and improved damaged liver tissue and decreased histological score. PDRN inhibited PI3K/Akt activation, and the enhancing effect of PDRN on cAMP level acted as a therapeutic mechanism for ALI.
As a result of this experiment, PDRN treatment improved liver function by increasing cAMP concentration in liver tissue and inhibiting apoptosis in ALI mice. In contrast, when the adenosine A2A receptor antagonist DMPX and the adenosine A2A receptor agonist PDRN were administered at the same time, the improvement of the liver function of PDRN disappeared. Thus, it could be suggested that PDRN has the potential to be selected as a therapeutic agent for ALI.
REFERENCESAhsan MK, Mehal WZ. Activation of adenosine receptor A2A increases HSC proliferation and inhibits death and senescence by down-regulation of p53 and Rb. Front Pharmacol. 2014;5:69
Buko V, Kuzmitskaya I, Kirko S, Belonovskaya E, Naruta E, Lukivskaya O, Shlyahtun A, Ilyich T, Zakreska A, Zavodnik I. Betulin attenuated liver damage by prevention of hepatic mitochondrial dysfunction in rats with alcoholic steatohepatitis. Physiol Int. 2019;106:323–334.
Chen M, Sun X, Wei W, Cucarella C, Martín-Sanz P, Casado M, Pi L, Ren B, Cao Q. Hepatic COX-2 expression protects mice from an alcohol-high fat diet-induced metabolic disorder by involving protein acetylation related energy metabolism. Alcohol. 2021;92:41–52.
Chen X, Bian M, Zhang C, Kai J, Yao Z, Jin H, Lu C, Shao J, Chen A, Zhang F, Zheng S. Dihydroartemisinin inhibits ER stress-mediated mitochondrial pathway to attenuate hepatocyte lipoapoptosis via blocking the activation of the PI3K/Akt pathway. Biomed Pharmacother. 2018;97:975–984.
Chen Z, Wu A, Jin H, Liu F. β-Sitosterol attenuates liver injury in a rat model of chronic alcohol intake. Arch Pharm Res. 2020;43:1197–1206.
Hwang L, Ko IG, Jin JJ, Kim SH, Kim CJ, Hwang JJ, Choi CW, Chang BS. Attenuation effect of polydeoxyribonucleotide on inflammatory cytokines and apoptotic factors induced by particulate matter (PM10) damage in human bronchial cells. J Biochem Mol Toxicol. 2021;35:e22635
Hyder MA, Hasan M, Mohieldein AH. Comparative levels of ALT, AST, ALP and GGT in liver associated diseases. Eur J Exp Biol. 2013;3:280–284.
Jiang Y, Luo Y, Chen X, Liu N, Hou J, Piao J, Song C, Si C, Hu W, Li X. Senkyunolide H protects PC12 cells from OGD/R-induced injury via cAMP-PI3K/AKT signaling pathway. J Ethnopharmacol. 2021;282:114659
Kim SH, Ko IG, Jin JJ, Hwang L, Kim BK, Baek SS. Study on the pathogenesis of liver injury caused by alcohol and drugs. J Exerc Rehabil. 2021a;17:319–323.
Kim SH, Ko YJ, Baek SS. Resistance exercise improves short-term memory through inactivation of NF-κB pathway in mice with Parkinson disease. J Exerc Rehabil. 2021b;17:81–87.
Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363–1370.
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ, Han JH, Lee S, Kim HI, Shin HP, Jeon JW. Polydeoxyribonucleotide exerts protective effect against CCl4-induced acute liver injury through inactivation of NF-κB/MAPK signaling pathway in mice. Int J Mol Sci. 2020a;21:7894
Ko IG, Jin JJ, Hwang L, Kim SH, Kim CJ, Jeon JW, Chung JY, Han JH. Adenosine A2A receptor agonist polydeoxyribonucleotide ameliorates short-term memory impairment by suppressing cerebral ischemia-induced inflammation via MAPK pathway. PLoS One. 2021;16:e0248689
Ko YJ, Kim BK, Ji ES. Treadmill exercise in obese maternal rats during pregnancy improves spatial memory through activation of phosphatidylinositol 3-kinase pathway in the hippocampus of rat pups. J Exerc Rehabil. 2020b;16:483–488.
Luo P, Zheng M, Zhang R, Zhang H, Liu Y, Li W, Sun X, Yu Q, Tipoe GL, Xiao J. S-Allylmercaptocysteine improves alcoholic liver disease partly through a direct modulation of insulin receptor signaling. Acta Pharm Sin B. 2021;11:668–679.
Mayer IA, Arteaga CL. The PI3K/AKT pathway as a target for cancer treatment. Annu Rev Med. 2016;67:11–28.
McCullough RL, McMullen MR, Das D, Roychowdhury S, Strainic MG, Medof ME, Nagy LE. Differential contribution of complement receptor C5aR in myeloid and non-myeloid cells in chronic ethanol-induced liver injury in mice. Mol Immunol. 2016;75:122–132.
Seitz HK, Bataller R, Cortez-Pinto H, Gao B, Gual A, Lackner C, Mathurin P, Mueller S, Szabo G, Tsukamoto H. Alcoholic liver disease. Nat Rev Dis Primers. 2018;4:16
Son MK, Ryu YL, Jung KH, Lee H, Lee HS, Yan HH, Park HJ, Ryu JK, Suh JK, Hong S, Hong SS. HS-173, a novel PI3K inhibitor, attenuates the activation of hepatic stellate cells in liver fibrosis. Sci Rep. 2013;3:3470
Sun YY, Zhao YX, Li XF, Huang C, Meng XM, Li J. β-arrestin 2 promotes hepatocyte apoptosis by inhibiting Akt pathway in alcoholic liver disease. Front Pharmacol. 2018;9:1031
Wahlang B, McClain C, Barve S, Gobejishvili L. Role of cAMP and phosphodiesterase signaling in liver health and disease. Cell Signal. 2018;49:105–115.
Wang R, Song F, Li S, Wu B, Gu Y, Yuan Y. Salvianolic acid A attenuates CCl4-induced liver fibrosis by regulating the PI3K/AKT/mTOR, Bcl-2/Bax and caspase-3/cleaved caspase-3 signaling pathways. Drug Des Devel Ther. 2019;13:1889–1900.
Fig. 1Changes of aspartate aminotransferase (AST) and alanine aminotransferase (ALT). Upper panel: Concentration of AST in serum. Lower panel: Concentration of ALT in serum. A, control group; B, alcoholic liver injury (ALI)-induced group; C, ALI-induced and polydeoxyribonucleotide (PDRN)-treated group; D, ALI-induced and PDRN with 3,7-dimethyl-1-propargylxanthine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the ALI-induced group. 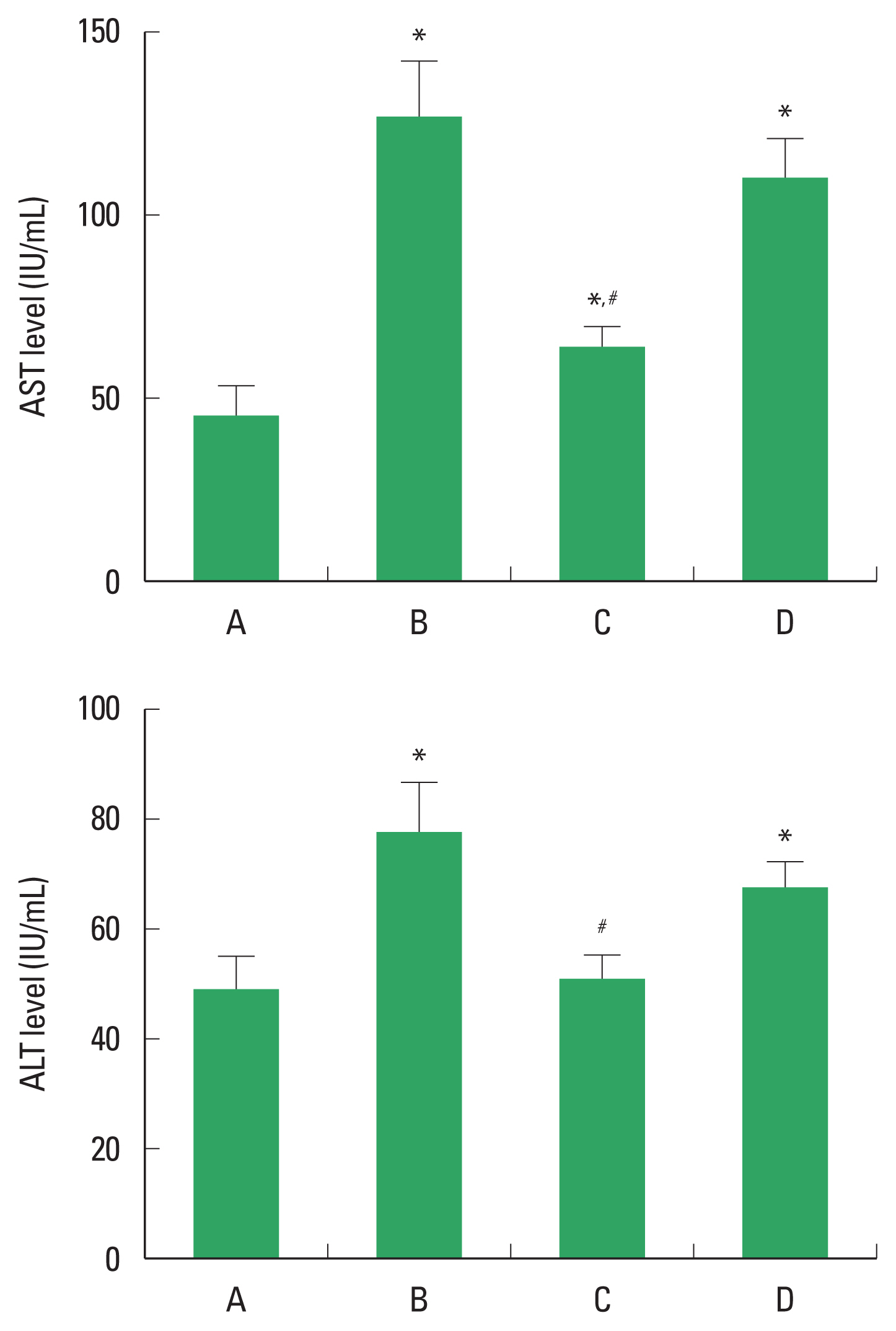
Fig. 2Changes of histology and histological score. Upper panel: the sections were stained with hematoxylin and eosin. The scale bar represents 150 μm. Lower panel: histological score in each group. A, control group; B, alcoholic liver injury (ALI)-induced group; C, ALI-induced and polydeoxyribonucleotide (PDRN)-treated group; D, ALI-induced and PDRN with 3,7-dimethyl-1-propargylxanthine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the ALI-induced group. 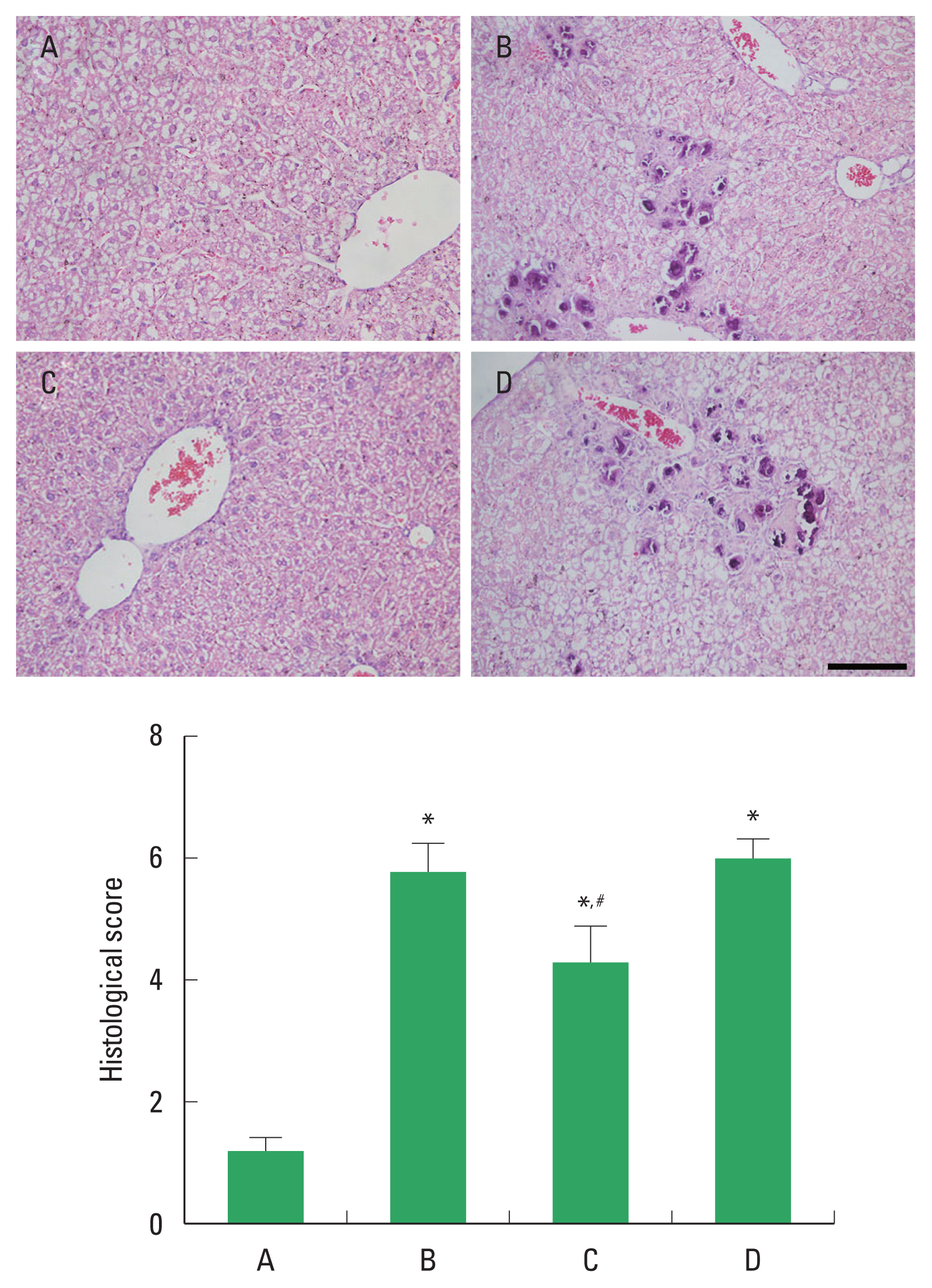
Fig. 3Changes of cyclic adenosine-3′,5′-monophosphate (cAMP) expression. Upper panel: concentration of cAMP in serum. Lower panel: concentration of cAMP in serum. A, control group; B, alcoholic liver injury (ALI)-induced group; C, ALI-induced and polydeoxyribonucleotide (PDRN)-treated group; D, ALI-induced and PDRN with 3,7-dimethyl-1-propargylxanthine-treated group. * Represents P<0.05 compared to the control group. #Represents P<0.05 compared to the ALI-induced group. 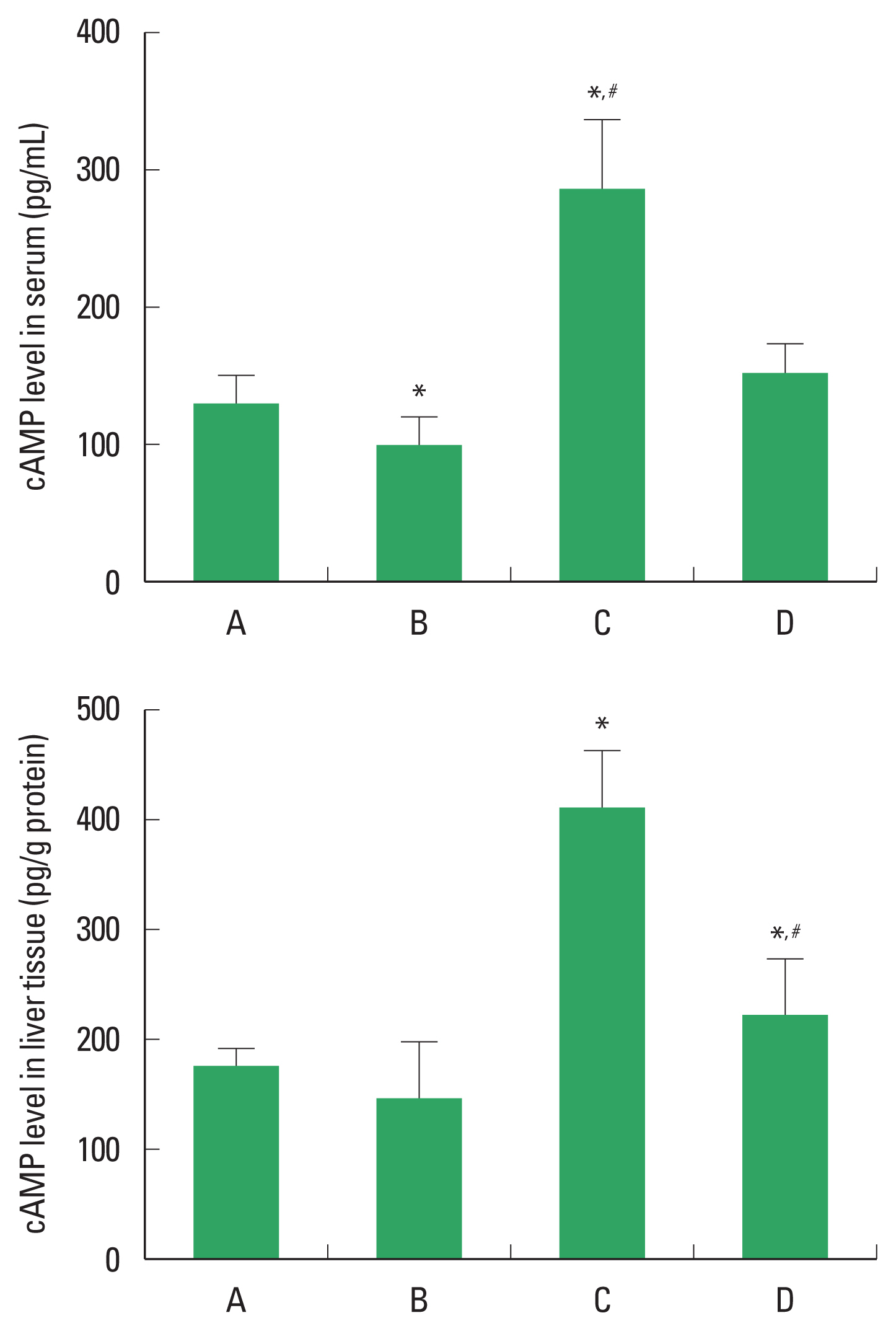
Fig. 4Changes of phosphatidylinositol 3-kinase/protein kinase B (PI3K/Akt) signaling pathway expression. Left panel: the relative ratio of phosphorylated Akt (p-Akt) to Akt in liver tissue. Right panel: the relative PI3K in liver tissue. A, control group; B, alcoholic liver injury (ALI)-induced group; C, ALI-induced and polydeoxyribonucleotide (PDRN)-treated group; D, ALI-induced and PDRN with 3,7-dimethyl-1-propargylxanthine-treated group. *P<0.05 compared to the control group. #P<0.05 compared to the ALI-induced group. 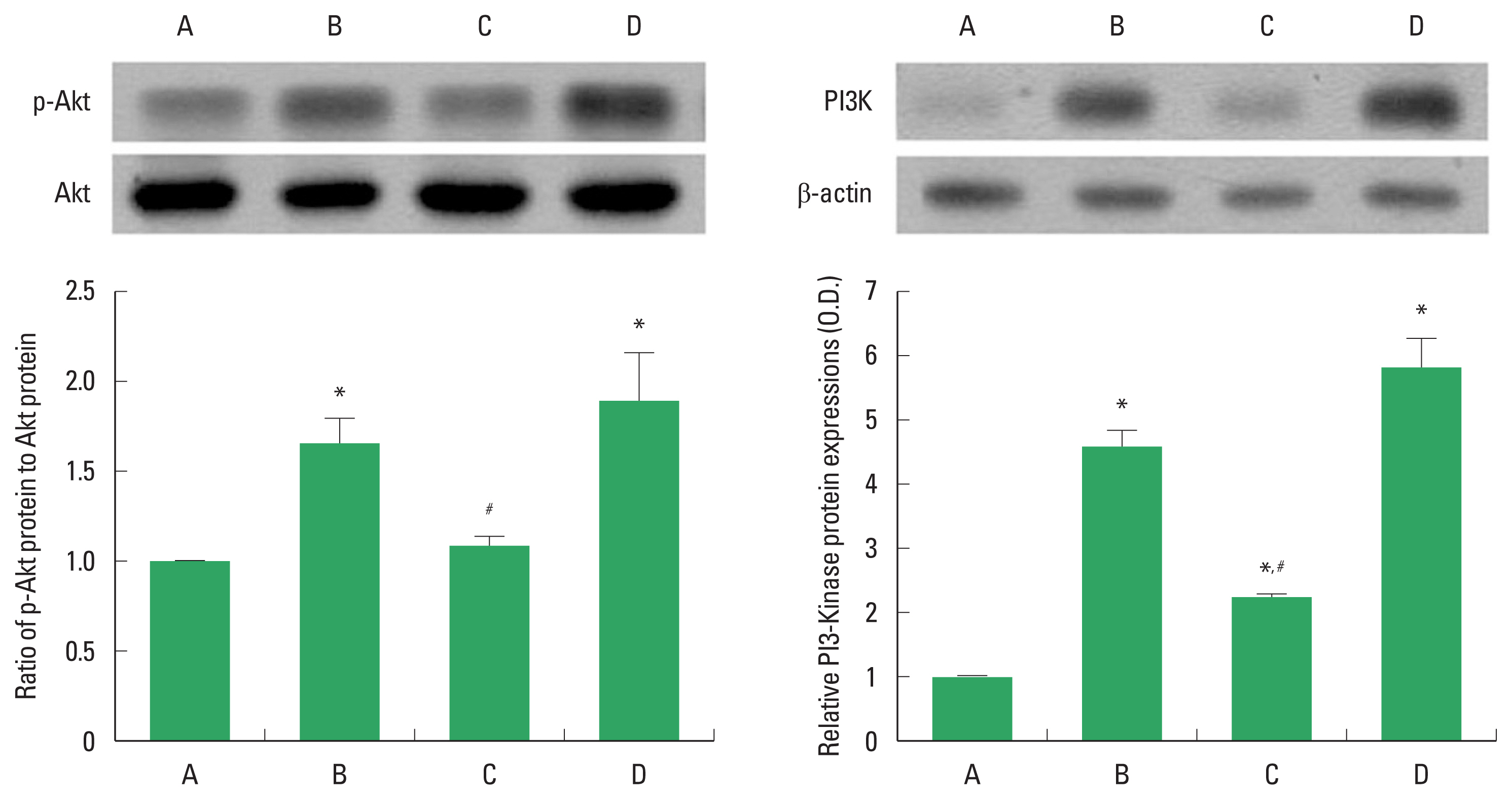
Table 1Primary and secondary antibodies in Western blot |
|
|||||||||||||||||||||||||||||||||||||||||||