INTRODUCTION
It has been reported that alcohol consumption in adolescence inhibits normal brain development, leading to a decrease in attention and concentration, as well as severe cognitive loss and visual impairment (McQueeny et al., 2009). The hippocampal volume of adolescents who consumed chronic alcohol was reduced by about 16% compared to normal adolescents (De Bellis et al., 2000). In addition, chronic alcohol intake was reported to have problems with language ability as well as deterioration of the adolescent’s learning ability and concentration (Brown et al., 2000).
Alcohol administration at a concentration of 6 g/kg increased the concentration of triglyceride in the mouse liver rapidly within 8 hours after ingestion, indicating the possibility of future fatty liver lesion expansion (Kaiser et al., 2009). As the intake of alcohol increased, the concentration of triglyceride in the liver increased higher, and it was reported that there was a high correlation between alcohol intake and fat accumulation in the liver (Donohue et al., 2012).
Muscle RING-finger protein-1 (MuRF1) is upregulated during skeletal muscle atrophy. Therefore, degradation of the myosin heavy chain, a major component of sarcomere, is an important mechanism for degradation of skeletal muscle under atrophic conditions. MuRF1 has been shown to be upregulated during denervation, glucocorticoid administration, immobilization, and casting (when a limb is cast for immobilization). All these treatments cause skeletal muscle atrophy (Olivé et al., 2015). As the intake alcohol concentration increased, the expression of proteolytic factors in muscle such as muscle atrophy F-box (MAFbx) and MuRF1 in skeletal muscle of adult rats increased (Vary et al., 2008).
Protein kinase B, also known as Akt, is the collective name of a set of three serine/threonine-specific protein kinases that play key roles in multiple cellular processes such as glucose metabolism, apoptosis, cell proliferation, transcription and cell migration. There are three different genes that encode isoforms of protein kinase B. These three genes are referred to as Akt1, Akt2, and Akt3. Akt1 is involved in the cell survival pathway by inhibiting the process of apoptosis. Akt1 can also induce protein synthesis pathways and thus is a key signaling protein in cellular pathways leading to skeletal muscle hypertrophy and general tissue growth. A mouse model in which the Akt1 gene is completely deleted exhibits growth retardation and increased spontaneous apoptosis in tissues such as the testis and thymus (Chen et al., 2001). Akt2 is an important signaling molecule in the insulin signaling pathway. The role of Akt3 is less clear, but appears to be primarily expressed in the brain.
The forkhead box O (FoxO) transcription factor plays a key role in an evolutionarily conserved pathway. FoxO proteins are involved in a variety of cellular functions and have clinical significance, including cell cycle arrest, cell differentiation, tumor suppression, DNA repair, lifespan, diabetic complications, immunity, wound healing, metabolic regulation and consequent treatment of several types of disease. Akt is a signaling pathway as a key regulator of FoxO that performs a variety of functions in organisms (Tia et al., 2018).
Chronic alcoholic myopathy is one of the most frequent and profound signs of chronic alcoholism. The disease is characterized by marked atrophy of motor muscles containing predominantly fibers expressing the myosin isoform of type I fast (Shenkman et al., 2013). Although many studies have been reported on the histologic and biochemical changes of skeletal muscle in adult and old rats according to alcohol intake, there are very few reports of changes in skeletal muscle according to alcohol intake using growing rats. Therefore, in this study, the effect of chronic alcohol intake for 4 weeks on glucose, total cholesterol, triglyceride and high-density lipoprotein cholesterol in serum level was measured and protein expression related to skeletal muscle atrophy in growing rats was studied.
MATERIALS AND METHODS
Animals and treatments
In this experiment, male Sprague-Dawley rats (Samtaco Bio Korea, Hwaseong, Korea) were used. A total of 18 experimental animals were purchased, and they were bred for 4 weeks in an animal breeding room, classified into a control group (n=9) and an alcohol intake group (n=9). The alcohol intake group was orally administered with alcohol (3-g/kg body weight) at 9 am every day, and the same amount of tap water was orally administered in the control group. This experiment was approved by the Research Ethics Committee of Eulji University (EU-2021-4-15).
Blood sampling and serum component analysis
After breeding for 4 weeks, the animals were anesthetized using ether, and blood was collected from the left ventricle using a disposable syringe and placed in a tube for serum separation. Blood was centrifuged at 700×g for 15 min using a refrigerated centrifuge at 4°C to separate serum. The concentrations of glucose, total cholesterol, triglyceride and high-density lipoprotein cholesterol in serum were analyzed by enzymatic method using a commercial kit (Asan Pharm., Seoul, Korea) with a spectrophotometer (Biochrom Ltd., Hollston, MA, USA).
Muscle tissue pretreatment
After removing all tendon tissues of the soleus muscle, cytoplasmic extract buffer was added 9 times the muscle tissue weight, and then homogenization was performed for about 10 sec using a polytron homogenizer (Biospec Products, Bartlesville, OK, USA). After homogenization, the supernatant was extracted by centrifugation at 10,000×g for 5 min. Meanwhile, the precipitate was centrifuged at 10,000×g for 5 min after adding nuclear extract buffer to extract the supernatant and used as a sample for protein expression. Protein concentration was quantified using the Bradford protein assay kit (Bio-Rad, Hercules, CA, USA) using bovine serum albumin as a standard concentration.
Western blotting
Western blot was performed using the soleus muscle. Proteins were electrophoresed on a 12% running gel. After electrophoresis, the gel was transferred to polyvinylidene diflouride membranes (Millipore, Bedford, MA, USA) at 4°C for 12 hr using a blotting device. Blocking was performed for 1 hr with 5% bovine serum albumin diluted with Tris-buffered saline in Tween-20 at room temperature. Primary antibodies against Akt, phosphorylated Akt (p-Akt), FoxO, phosphorylated FoxO (p-FoxO) (Cell Signaling, Beverly, MA, USA), MuRF1, p38, and phosphorylated p38 (p-p38) (Abcam, Cambridge, MA, USA) were prepared using 5% bovine serum at room temperature. The secondary antibody was reacted with horseradish peroxidase-conjugated anti-goat IgG (Santa Cruz Biotechnology, Santa Cruz, CA, USA) or horseradish peroxidase-conjugated anti-rabbit IgG (Santa Cruz Biotechnology) for 1 hr, respectively. Thereafter, each protein band was identified using an enhanced chemiluminescence kit (GE Healthcare, Buckinghamshire, UK).
Data analysis
The data processing was analyzed using SPSS ver. 16.0 (SPSS Inc., Chicago, IL, USA) and all data was shown as the mean standard error of the mean. Independent specimens were performed to verify the difference between groups, and the statistical significance level was set to P<0.05 level.
RESULTS
Effect of alcohol intake on blood composition
As a result of ingestion of alcohol for 4 weeks, the changes of glucose, total cholesterol, triglyceride, and high-density lipoprotein cholesterol in serum are presented in Table 1. Only triglyceride concentration in the alcohol intake group was increased when compared to the control group.
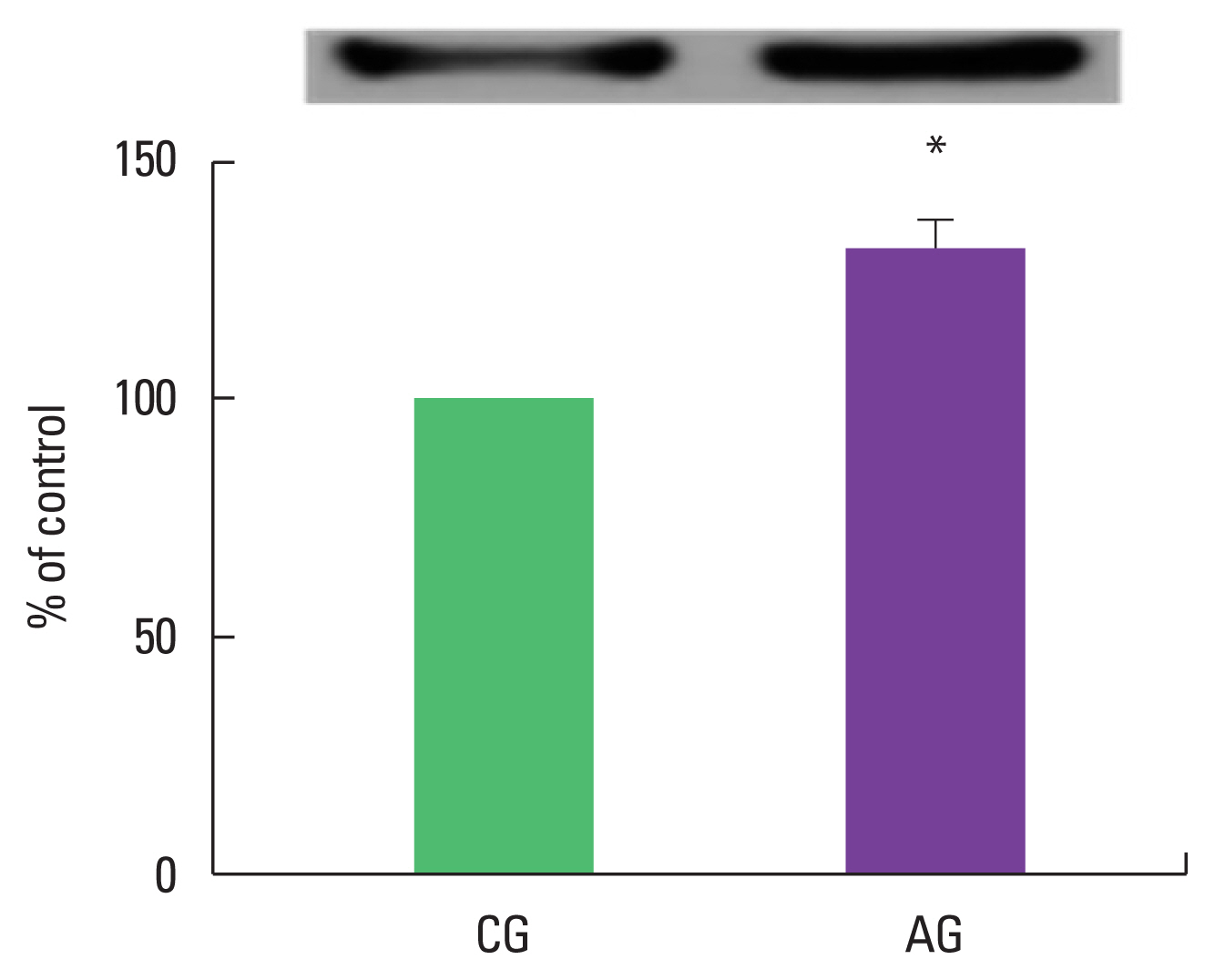
Effect of alcohol intake on MuRF1 expression
As a result of ingestion of alcohol for 4 weeks, MuRF1 protein expression in the muscle was high as 132.4%±6.02% in the alcohol intake group when the control group was set at 100% (Fig. 1).
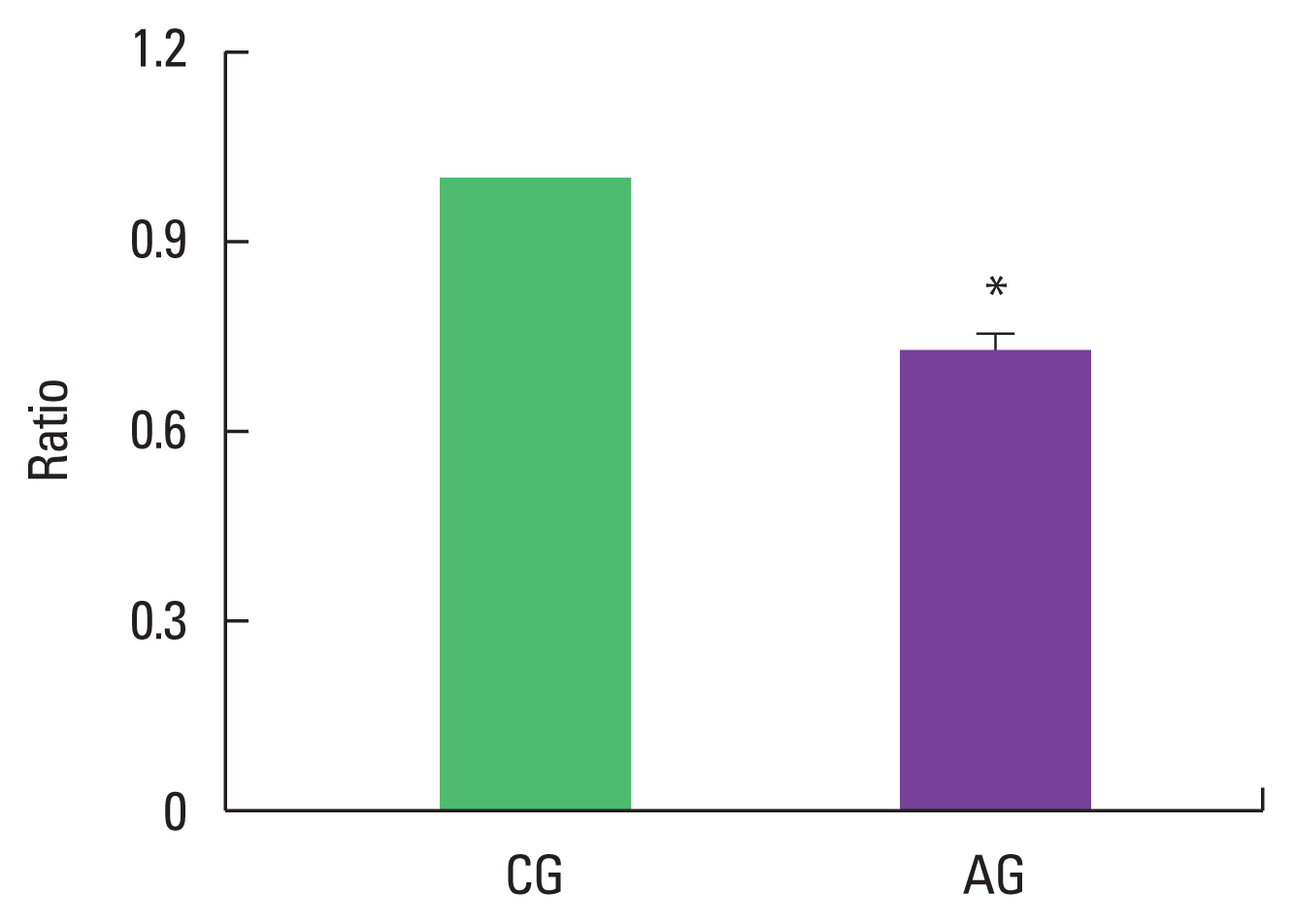
Effect of alcohol intake on ratio of p-Akt/Akt expression
As a result of ingesting alcohol for 4 weeks, the change in p-Akt/Akt ratio of the muscle was low as 0.73±0.03 in the alcohol intake group when the control group was set to 1 (Fig. 2).
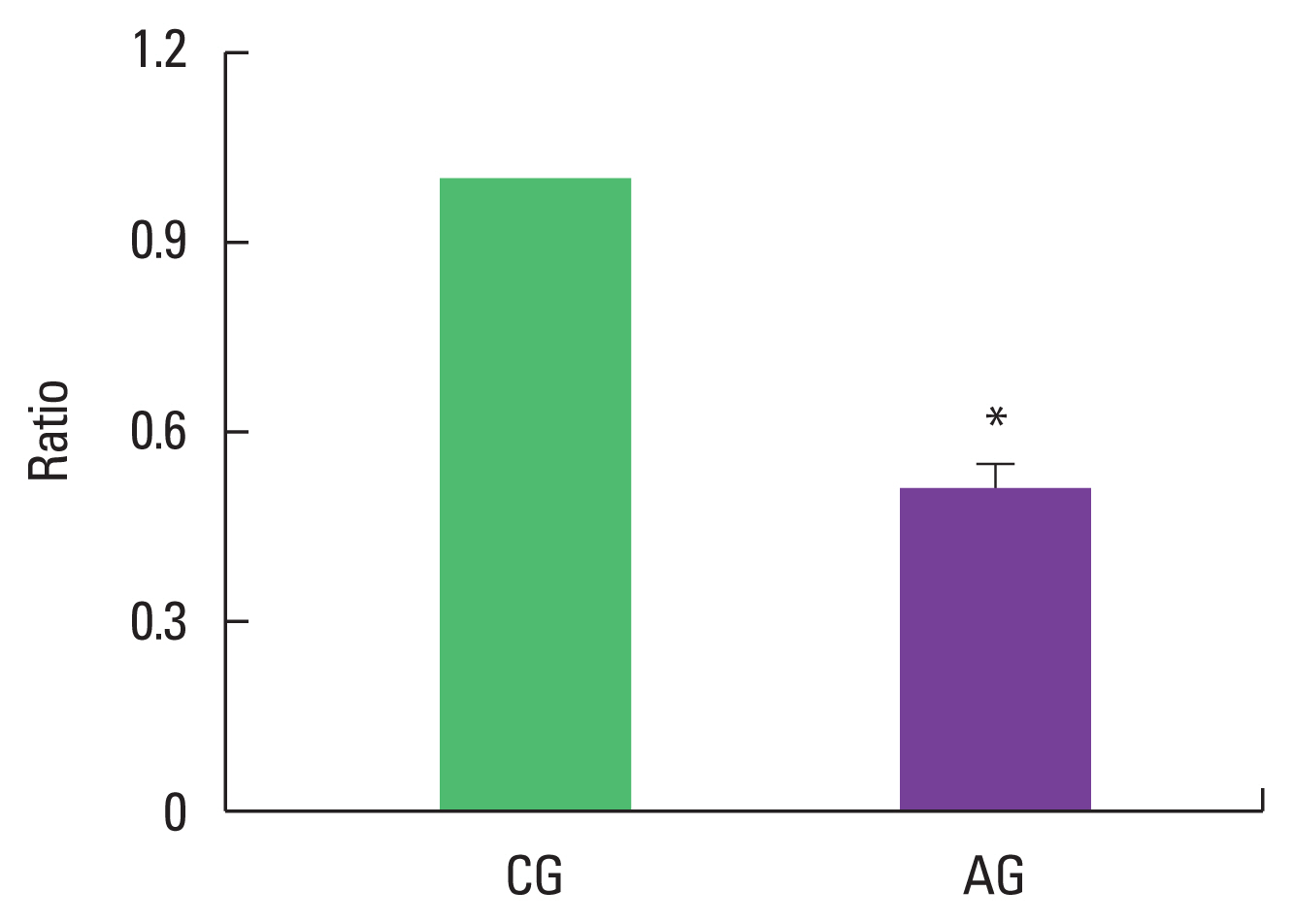
Effect of alcohol consumption on ratio of p-FoxO/FoxO expression
As a result of ingesting alcohol for 4 weeks, the change in p-FoxO/FoxO ratio of the muscle was as low as 0.51±0.04 in the alcohol intake group when the control group was set to 1 (Fig. 3).
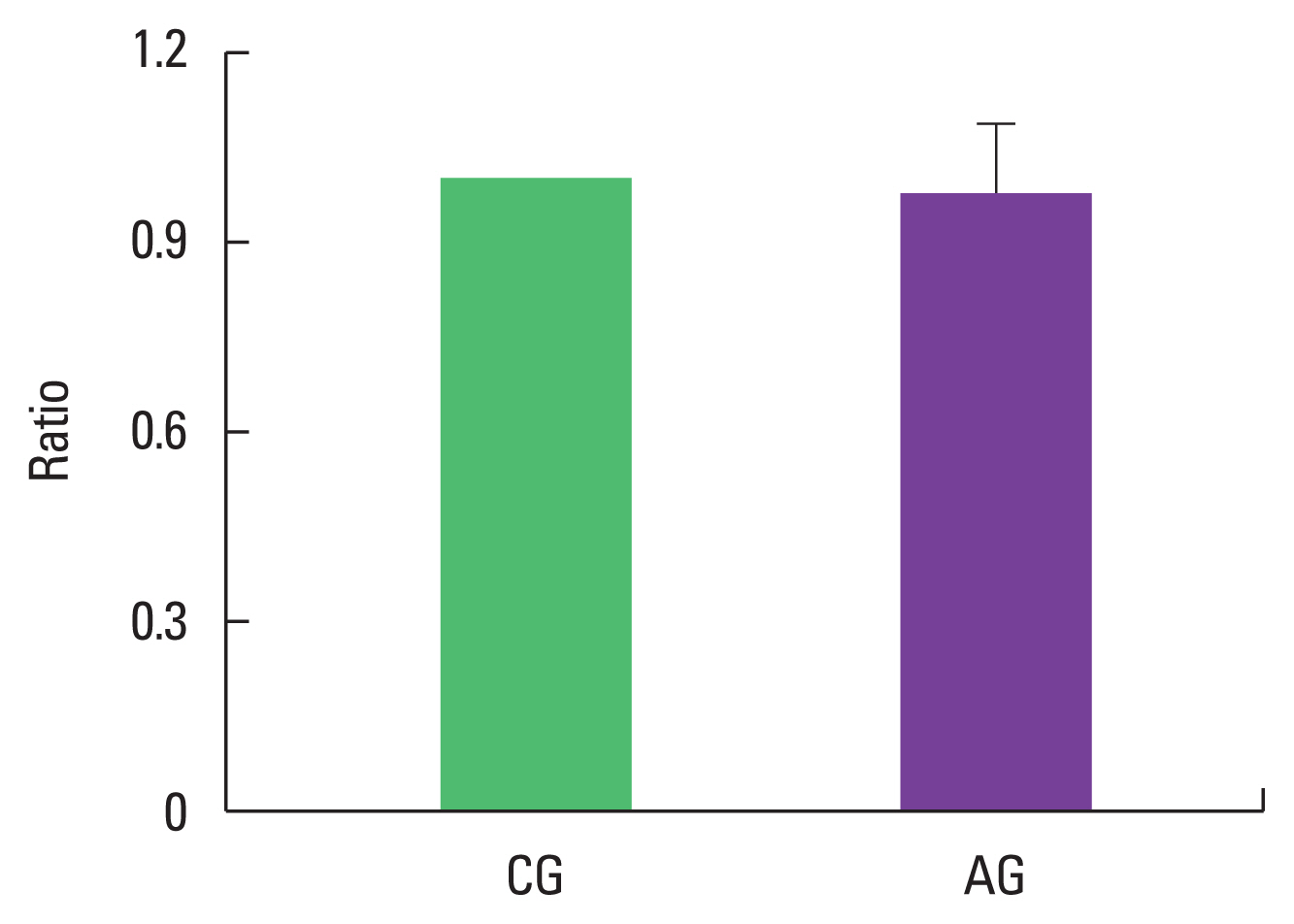
Effect of alcohol consumption on ratio of p-p38/p38 expression
As a result of ingesting alcohol for 4 weeks, the change in p-p38/p38 ratio of the muscle was 0.98±0.11 in the alcohol intake group when the control group was set to 1, indicating no difference (Fig. 4).
DISCUSSION
In the present study, alcohol intake for 4 weeks increased the triglyceride concentration in the blood. In an animal experiment on rats chronically ingesting 36% alcohol for 4 months, fatty acid metabolism in the liver was reduced by an increase in serum triglyceride concentration (Romero et al., 2014).
In the present study, MuRF1 protein expression in the alcohol intake group was significantly increased compared to the control group. It was reported that MuRF1 and MAFbx expression increased in an experiment in which skeletal muscle atrophy was induced (Bodine et al., 2001). Vary et al. (2008) showed that the expression of MuRF1 protein was significantly increased in an experiment in which skeletal muscle was treated with alcohol at a concentration of 75 mmol/kg for 24 hr in an animal experiment.
In the present study, p-Akt/Akt ratio in the alcohol intake group was significantly decreased compared to the control group. Akt has been reported to play an important role in the growth of tissues such as bone and muscle tissue (Peng et al., 2003). Blaauw et al. (2009) reported that phosphorylation of Akt acts as an important factor in muscle tissue hypertrophy in a study using Akt transgenic mice. As a result of treatment with insulin growth factor-1 in a study using myocytes, and in a study using the gastrocnemius muscle in the growth phase, it was reported that the expression of p-Akt and p-insulin growth factor-1 was promoted, thereby promoting muscle fiber hypertrophy (Stitt et al., 2004). It has been suggested that Akt has a direct association with tissue growth (Stitt et al., 2004). Various molecular biological approaches are also being made in relation to alcohol intake. As a result of ingestion of alcohol (3.5 g/kg of body weight) in rats, phosphorylation of Akt in brain was reduced (Laguesse et al., 2017).
In the present study, p-FoxO/FoxO ratio in the alcohol intake group was significantly decreased compared to the control group. Insulin inhibits FoxO transcription factors, which down-regulate the expression of genes involved in metabolism, cell cycle arrest, and apoptosis. FoxO turns on a feedforward loop that is relayed by p38 and serves to amplify both Akt activation and FoxO repression. In conclusion, key signaling pathways are activated in hepatocytes via nuclear FoxO (Naïmi et al., 2007). Overexpression of the predominantly negative form of FoxO suppressed gene expression associated with ethanol-induced autophagy and enhanced ethanol-induced apoptosis in primary hepatocytes, suggesting that FoxO is a key factor in regulating ethanol-induced autophagy and cell survival. (Ni et al., 2013).
In the present study, p-p38/p38 ratio in the alcohol intake group was not changed compared to the control group. Phosphorylation of p38 was increased in the skeletal muscle of rats weighing 400 g, in which 36% of daily total calories were replaced with alcohol for 12 weeks, and at the same time, the muscle cross-sectional area and muscle weight were decreased (Clary et al., 2011). However, there was no change in the phosphorylation of p38 in this experiment.
As a result of this experiment, chronic alcohol intake in growing rats promoted muscle protein degradation by increasing MuRF1 protein expression and inhibited skeletal muscle growth by decreasing the p-Akt/Akt ratio and p-FoxO/FoxO ratio. Therefore, it was shown that alcohol intake induces muscle atrophy.