AbstractRegular exercise is associated with the production of small amounts of oxidative stress which might promote individual antioxidant capacity contributing to favorable training effects potentially interrelated with skeletal muscle strength. Therefore, the present study was aimed at evaluating effects of an 8-week Qigong exercise training on muscle strengths associated with responses of oxidative stress and antioxidants in young sedentary females. A total of 41 sedentary women were allocated to the Qigong exercise group (QG, N=20) or to the control group (CG, N=21). After 8 weeks of Qigong training, back and leg strength was significantly improved compared to baseline and the CG (P<0.05). Plasma oxidative stress levels were reduced and total antioxidant capacity was enhanced in the QG compared to the CG (P<0.05). Correlation analyses revealed that improvements in muscle strength (including both groups) were associated with changes in the levels of oxidative stress (reduction) and antioxidants (elevation). The presented findings indicate that strength training effects seem at least partly to be interrelated with alterations of the oxidant-antioxidant balance generated by the 8-week Qigong training in young sedentary females.
INTRODUCTIONQigong exercise training was shown to elicit beneficial effects on physical performance in women (Sakata et al., 2008). This type of exercise is a traditional Chinese exercise, consisting of isometric, aerobic, isotonic, relaxing, and meditative movements (Larkey et al., 2009; Tsang et al., 2003). It may result in improved trunk strength and movement abilities in the elderly (Wolf et al., 1996). Additionally, it was demonstrated to improve quality of life in patients suffering from chronic illnesses, i.e., diabetes mellitus, dyslipidemia, and cancer (Cavegn and Riskowski, 2015; Chuang et al., 2017; Pan et al., 2016). For instance, Qigong exercise enhanced balance and fitness as well as somatosensation in diabetes mellitus patients (Cavegn and Riskowski, 2015), decreased fatigue, improved sleep quality along with increased white blood cell counts and hemoglobin levels in non-Hodgkin lymphoma patients (Chuang et al., 2017). Moreover, practicing such exercises may also impact on lipid metabolism, but information on most appropriate intensity and duration is still lacking (Pan et al., 2016). Endurance exercise is associated with enhanced oxygen consumption also resulting in elevated production of reactive oxygen species (ROS) (Alessio, 1993).
On the other hand, current studies show that regular exercise training will promote upregulation of the antioxidant capacity (Clarkson and Thompson, 2000; Elosua et al., 2003; Evelo et al., 1992). Similar results were demonstrated with the use of other traditional Chinese exercises. With respect to previous studies, Tai Chi and Qigong effects have mainly been evaluated in middle aged and older people (Goon et al., 2009; Li et al., 2001; Thornton et al., 2004; Wolf et al., 1996; Wolfson et al., 1996). The assumption that the outcomes of one population could be transferred to another population through the same intervention may not hold true. For instance, Tai Chi or Qigong exercise affects more beneficially cardiovascular performance of older than younger adults (Zhuo et al., 1984). Gender differences may also occur with regard to the motivation for exercise. Gordon-Larsen et al. (2004) reported less physical activity in females than males and sedentary behavior trends in adolescence will continue to persist into adulthood. Qigong exercise training for 4 weeks improved balance in young women (González López-Arza et al., 2013). Huang et al. (2014) demonstrated that Tai Chi training for 8 weeks reduced oxidative stress levels in young sedentary females, however, the lack of control group limits firm conclusions. There are several studies suggesting improved antioxidant capacity and anti-inflammatory responses following Tai Chi training (Mendoza-Nunez et al., 2014; Rosado-Perez et al., 2013). It is likely that the small amount of ROS generation from moderate-intensity exercise stimulates signaling pathways contributing to upregulation of antioxidant enzymes (Gomez-Cabrera et al., 2008) and the improved force generation (Andrade et al., 2001).
The relationship between changes in oxidative stress/total antioxidant capacity (TAC) and skeletal muscle strength resulting from regular Qigong training has been rarely studied particularly in the population of young sedentary females. Thus, the present study was aimed to determine the impact of Qigong training over 8 weeks on those parameters in young and untrained women. We hypothesized that Qigong training will modulate the balance between oxidative stress and TAC potentially associated with the improvement in muscle strength of the back and legs and trunk flexibility.
MATERIALS AND METHODSParticipantsVoluntary young female participants were enrolled from students of Burapha University, Bangsean, Chonburi province, Thailand. Inclusion criteria were an age range between 21 to 23 years, being nonsmoker, not drinking alcohol, not being physically active on a regular basis (more than once per month), and not taking antioxidant supplementation. Physical activity records were used to assess sedentary lifestyle (Tremblay et al., 2017). Participants of the CG were advised to continue their lifestyle throughout the study period. Exclusion criteria were any disease that would be associated with a health risk when performing the Qigong training. The PAR-Q was used to assess a potential health risk associated with exercise (American College of Sports Medicine, 2018).
Due to the relatively long intervention period, a quasi-experimental design was employed for allocating 43 female undergraduate students to the Qigong training group (QG, N=22) and the control group (CG, N=21). The study was reviewed and approved by Burapha University Human Institutional Review Board (021/2563) and Thai Clinical Trial Registry (TCTR20200805003). The work was done in accordance with the appropriate institutional review body and carried out with the ethical standards set forth in the Helsinki Declaration of 1975.
Qigong exercise intervention and blood sample collectionQigong exercise consisted of gentle, concentrated movements together with breathing, relaxation, and carefulness by the use of 18 typical movements modified according to Taiji Qigong by Robinson (2017) and Ladawan et al. (2017). The QG was leaded by an instructor who continued Qigong exercise training for 8 years. The Qigong exercise training started with a 2-min warm-up by stretching, followed by the 18 movements of Qigong exercise for 60 min and was finished with 2 min of stretching for cool down. The Qigong training was performed once a day, 5 days per weeks over 8 weeks.
Blood samples were taken from antecubital veins 3 times at the same time and days for both QG and CG. Subjects were overnight fasting before the blood collections taken at around 7 a.m. Blood samples of both groups were collected at baseline (PRE), and the next morning after finishing the first day of exercise (or observation in the CG), and were defined as ACUTE. The 3rd determination was done the next morning after completing 8 weeks of Qigong exercise training (or 8 weeks of observation in the CG) defined as POST (Fig. 1).
Assessment of back and leg strengthThe back and leg muscles strength were assessed using an electronic dynamometer (T.K.K. 5102 Back-D, Takei Scientific Instruments Co., Tokyo, Japan). Subjects stood on the board of the dynamometer with their feet about 20–30 cm apart. For determining back strength, they grasped the grips with both hands without bending their knees, set at 30° of hip flexion then adjusted the length of the chains and gently strained the back muscle (Oh et al., 2012). The leg muscle strength was determined with subjects bended their knees about 130°–140°, while grasping the grips with both hands in stretch position. The chain was held at an appropriate knee angle. The measurement of leg strength was determined while subjects slowly but powerfully stretched the legs without using back muscle (Arslan, 2005). Two attempts were made for each test with a 1-min rest attempts. Measured values were averaged (unit in kg) and divided by the individual body mass (kg).
Assessment of trunk flexibilityThe trunk flexibility was determined by use of the sit and reach test. Participants sat on the floor and placed feet against the box (Baseline 12-1085 Sit n’ Reach Trunk Flexibility Box-Standard, Fabrication Enterprises, Inc., White Plains, NY, USA) no wider than 8 inches apart with hips, back and head against a wall. Legs must be extended with back of knees touching the floor. Subjects were instructed to place one hand on top of the other and to reach as far as possible. The back of the knees must stay flat on the floor. There should be no lunging, bobbing, or forced assistance. The stretch has to be held for 1 sec (Trajkovic et al., 2020). Two attempts were used to determine the trunk flexibility and values were averaged (unit in cm).
Determination of TAC as ferric reducing antioxidant power assayThe ferric reducing antioxidant power (FRAP) assay was modified from Benzie and Strain (1996) and Frei et al. (1989), then employed into 96 well plate reader. Plasma samples were diluted into 10 times with normal saline. In our evaluations, FRAP concentrations were ranged from approximately 0 to 1,000 μM. Each reaction contained 75 μL of the FRAP color solution (12.5 mL of 300 mmol/L acetate buffer pH 3.6, 1.25 mL of 10.0 mmol/L 2,4,6-tripyridyl-s-triazine solution, 1.25 mL of 20.0 mmol/L FeCl3.6 H2O solution), and 20 μL of the samples into duplicate wells in the plate. The resulting mixture was vigorously shaken and then incubated at 25°C for 30 min and the increase in absorbance at 560 nm was measured and compared with the ferrous chloride standard and ascorbic acid as control.
Determination of catalase enzyme activityThe catalase enzyme activity (CAT) activity assay was measured using spectrophotometric determination of hydrogen peroxide (H2O2) which form stable complex with ammonium molybdate that absorbs at 405 nm (Goth, 1991). Briefly, 1 mL of substrate (65 μmol per mL H2O2 in 60 mmol/L phosphate buffer, pH 7.4) was incubated with 50 μL of serum at 37°C for 60 sec. The enzymatic reaction was stopped with 1.0 mL of 32.4 mmol/L ammonium molybdate ((NH4)6 Mo7O24·4 H2O) and the yellow complex of molybdate and hydrogen peroxide is measured at 405 nm against reagent blank. The serum CAT activity was linear up to 100 kU/L. If the CAT activity exceeded 100 kU/L, the serum should be diluted with phosphate buffer. One unit of CAT decomposes 1 μmole of hydrogen peroxide/l min under assay conditions. The CAT activities are expressed as kilo unit per liter (kU/L).
Determination of oxidative stress as malondialdehyde levelMalondialdehyde (MDA) was measured by thiobarbituric acid (TBA) reactive substance assay modified from Nielsen et al. (1997) and Tsai et al. (1993). A 1 mL of 1:2 diluted plasmas was added with 50 μL of 0.1-mM butylated hydroxyl toluene, 500 μL of 5-mM ethylenediamine tetraacetic acid (EDTA), 1 mL of 8.1% (w/v) sodium dodecyl sulfate (SDS), 1 mL of 10% (w/v) trichloroacetic acid, and 1.5 mL of 0.67% (w/v) TBA. After that the mixture was incubated at 95ºC for 30 min, then dipped into water for 5 min. Later, it was centrifuged at 3,000 rpm for 15 min at room temperature. The supernatant was transferred to a glass cuvette for detecting absorbance at 532 nm. Tetraethoxypropane was used as standard and set the concentration range between 0.25–2 μM.
Determination of blood uric acid and albuminBlood samples were transferred to EDTA tube for blood uric acid and albumin. Then, samples were delivered on ice and immediately measured by National Healthcare system (Samitivej Chonburi Hospital). Albumin was analyzed using automated machine (Cobas Cobas c 311/501 analyzers, Roche Diagnostics GmbH, Sandhofer Strasse, Manheim, Germany). Blood uric acid was determined using the peroxide reacts in the presence of peroxidase, N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3-methylaniline, and 4-aminophenazone to form a quinone-diimine dye. The intensity of the red color formed is proportional to the uric acid concentration and is determined photometrically (Cobas Cobas c 311/501 analyzers, Roche Diagnostics GmbH).
Data analysisData analyses were performed by using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). The Kolmogorov–Smirnov test was used to test normality. Normally distributed data were expressed as mean±standard deviation. Not normally distributed data are reported as median (interquartile range). The main outcomes of physical performances (back and leg strength, and trunk flexibility) for changes from PRE to POST within each group were determined using paired t-test or Wilcoxon signed-rank test as appropriated. Student t-test or Mann–Whitney U-test was performed to compare mean differences of POST minus PRE between the exercise group and the control group. Interactions (time×group) were computed using analysis of variance with repeated measurements and two factors, in which group (QG or CG) was fixed factor and training time points (PRE, ACUTE, and POST) was repeated factor. A P<0.05 was considered statistically significant.
A power calculation for sample estimation was based on leg strength with regard to the study of Song et al. (2014), comparing before to after Tai chi exercise training at 4 months. With an alpha at 0.05 and power at 0.90 using paired t-test, the sample size was 20 participants per group (G*power 3.1).
RESULTSPRE, (ACUTE) and POST-measurements were completed by 20 participants of the QG and 21 participants of the CG. Two participants of the QG dropped out because they were not able to continue their training as indicated in the flow diagram demonstrating the study progress (Fig. 2). Some participants from the QG changed their exercise time from the morning to the evening. Baseline characteristics did not differ between QG and CG (Table 1).
Physical performancesBack and leg strength values were significantly improved after Qigong training (POST) and those changes were also significantly different compared to the CG (all P<0.05) (Table 2). Trunk flexibility was significantly enhanced only after Qigong training (POST) but did not differ compared to the CG (Table 2).
Plasma oxidative stress and antioxidantsSignificant interaction effects (time×group) were found with regard to the MDA, albumin, and TAC levels (P<0.05) (Table 3). Compared to the QG, MDA and albumin levels were increased and TAC levels were decreased (postintervention) in the CG.
DISCUSSIONThe main findings of the present study demonstrate improved back and leg strength in the QG compared to the CG and that alterations of the strength levels (within both groups) were negatively correlated with changes in oxidative stress and positively with antioxidant capacity. These results indicate that strength training effects may at least partly be interrelated with alterations of the oxidant-antioxidant system induced by Qigong training in young previously sedentary females.
Effects of ROS on excitation-contraction coupling were shown by Reid et al. (1993) who demonstrated that skeletal muscle bundles decreased force generation with exogenous catalase incubation and in contrast, exogenous hydrogen peroxide incubation induced increased force production. Therefore, oxidative stress, at least in low concentration, appears to be a prerequisite for beneficial training effects opposed to large level of ROS which may induce inhibition of function (Radak et al., 2005). Qigong training prevented the elevation in MDA levels and the reduction in TAC levels as observed in the CG.
The favorable effects of Qigong training on strengths are consistent with previous studies demonstrating significant improvements of knee extension strength after Tai chi training in elderly subjects (Christou et al., 2003; Lan et al., 2000; Song et al., 2014; Wolfson et al., 1996). Additionally, Hart and Tracy (2008) reported that Yoga training for 8 weeks significantly increased knee strength in young adults. The Qigong posture is similar to Tai chi where exercises are carried out in semisquat posture with slow movements and load shifting of the body. The changing sequence of concentric and eccentric contractions are primarily involving the lower extremities in closed kinetic chain exercise (Christou et al., 2003; Lan et al., 2000). Mechanisms related to improved strength and performance of skeletal muscles following resistance training include increase of muscle cross-section and changes in recruitment and firing rates of motor unit types (Carroll et al., 2001; Tracy et al., 2004). Based on our observations, changes in the oxidant-antioxidant balance may also have modified strength development due to Qigong training. The observations of elevated TAC and reduced oxidative stress and albumin levels after Qigong training are well in accordance with results from Rosado-Perez et al. (2013) and Mendoza-Nunez et al. (2014) who demonstrated that Tai Chi exercise over 6 months increased TAC, while oxidative stress (ROS) was reduced (Rosado-Perez et al., 2013). The slight decrease of albumin levels in the QG compared to the CG seems surprising. Albumin is one of the protein thiols that is abundant in plasma (Kundi et al., 2015). Gol et al. (2019) indicated that the total thiol pool that stands for antioxidant capacity is of insufficient function in sedentary people. Thus, in conditions associated with increasing oxidative stress, stimulation of the antioxidant defense and protein thiols may be not sufficiently effective in subjects with sedentary lifestyle (Gol et al., 2019; Tebay et al., 2015).
The light to moderate stimuli of Qigong exercise might induce small oxidative damage resulting in upregulation of the antioxidant systems and repair mechanism (Evans and Cannon, 1991). This is consistent with current studies suggesting that regular exercise activates antioxidant protection (McArdle and Jackson, 2000) and oxidative repair response (Sato et al., 2003; Wittwer et al., 2004). Results of the presented correlation analysis reveal a positive relationship between the Qigong training associated improvements in the antioxidant capacity and muscular strength enhancement while MDA levels were negatively related to strength development. Low concentration of hydrogen peroxide in skeletal muscle enhances Ca2+ discharge from the sarcoplasmic reticulum and force generation while large amount of hydrogen peroxide develops a reduction of force production (Andrade et al., 2001).
If confirmed in more large-scaled studies, these findings will not only have implication on the understanding of underlying mechanisms responsible for Qigong training effects but also for the appropriate use of antioxidants with exercise training for improving muscle strengths. Some limitations have to be mentioned. First, participants were not randomly assigned to the intervention or CG due to potential time constraints. However, based on their characteristics (Table 1) no differences between groups did exist. Furthermore, we did not strictly control diet during the study period but participants were asked not to change diet which, based on our observations, may have been actually followed. Nevertheless, strengths of the current study include the relatively large sample of homogenous sedentary females and the properly monitored exercise training.
In conclusion, the presented findings demonstrate improved back and leg strength in previously sedentary young females after 8 weeks of Qigong training when compared to controls. Strength training effects seem at least partly to be interrelated with alterations of the oxidant-antioxidant balance generated by Qigong training. Due to the well-known health effects of strength improvements and the easy and safely to perform Qigong training, these results may have important implications on the promotion of lifestyle changes in young sedentary individuals.
ACKNOWLEDGMENTSThis work was support by the Faculty of Allied Health Science, Burapha University grant in the year 2018.
REFERENCESAmerican College of Sports Medicine. ACSM’s guidelines for exercise testing and prescription. 10th ed. Philadelphia (PA): Wolters Kluwer; 2018.
Andrade FH, Reid MB, Westerblad H. Contractile response of skeletal muscle to low peroxide concentrations: myofibrillar calcium sensitivity as a likely target for redox-modulation. FASEB J. 2001;15:309–311.
Arslan C. Relationship between the 30-second wingate test and characteristics of isometric and explosive leg strength in young subjects. J Strength Cond Res. 2005;19:658–666.
Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76.
Carroll TJ, Riek S, Carson RG. Neural adaptations to resistance training: implications for movement control. Sports Med. 2001;31:829–840.
Cavegn EI, Riskowski JL. The effects of Tai Chi on peripheral somatosensation, balance, and fitness in Hispanic older adults with type 2 diabetes: a pilot and feasibility study. Evid Based Complement Alternat Med. 2015;2015:767213
Christou EA, Yang Y, Rosengren KS. Taiji training improves knee extensor strength and force control in older adults. J Gerontol A Biol Sci Med Sci. 2003;58:763–766.
Chuang TY, Yeh ML, Chung YC. A nurse facilitated mind-body interactive exercise (Chan-Chuang qigong) improves the health status of non-Hodgkin lymphoma patients receiving chemotherapy: randomised controlled trial. Int J Nurs Stud. 2017;69:25–33.
Clarkson PM, Thompson HS. Antioxidants: what role do they play in physical activity and health? Am J Clin Nutr. 2000;72:637S–646S.
Elosua R, Molina L, Fito M, Arquer A, Sanchez-Quesada JL, Covas MI, Ordoñez-Llanos J, Marrugat J. Response of oxidative stress biomarkers to a 16-week aerobic physical activity program, and to acute physical activity, in healthy young men and women. Atherosclerosis. 2003;167:327–334.
Evans WJ, Cannon JG. The metabolic effects of exercise-induced muscle damage. Exerc Sport Sci Rev. 1991;19:99–125.
Evelo CT, Palmen NG, Artur Y, Janssen GM. Changes in blood glutathione concentrations, and in erythrocyte glutathione reductase and glutathione S-transferase activity after running training and after participation in contests. Eur J Appl Physiol Occup Physiol. 1992;64:354–358.
Frei B, England L, Ames BN. Ascorbate is an outstanding antioxidant in human blood plasma. Proc Natl Acad Sci U S A. 1989;86:6377–6381.
Gol M, Ozkaya B, Yildirim C, Bal R. Regular exercise, overweight/obesity and sedentary lifestyle cause adaptive changes in thiol-disulfide homeostasis. An Acad Bras Cienc. 2019;91:e20180547
Gomez-Cabrera MC, Domenech E, Vina J. Moderate exercise is an antioxidant: upregulation of antioxidant genes by training. Free Radic Biol Med. 2008;44:126–131.
González López-Arza MV, Varela-Donoso E, Montanero-Fernández J, Rodríguez-Mansilla J, González-Sánchez B, González López-Arza L. Qigong improves balance in young women: a pilot study. J Integr Med. 2013;11:241–245.
Goon JA, Aini AHN, Musalmah M, Anum MYY, Nazaimoon WMW, Ngah WZW. Effect of Tai Chi exercise on DNA damage, antioxidant enzymes, and oxidative stress in middle-age adults. J Phys Act Health. 2009;6:43–54.
Gordon-Larsen P, Nelson MC, Popkin BM. Longitudinal physical activity and sedentary behavior trends: adolescence to adulthood. Am J Prev Med. 2004;27:277–283.
Goth L. A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta. 1991;196:143–151.
Hart CE, Tracy BL. Yoga as steadiness training: effects on motor variability in young adults. J Strength Cond Res. 2008;22:1659–1669.
Huang XY, Eungpinichpong W, Silsirivanit A, Nakmareong S, Wu XH. Tai chi improves oxidative stress response and DNA damage/repair in young sedentary females. J Phys Ther Sci. 2014;26:825–829.
Kundi H, Ates I, Kiziltunc E, Cetin M, Cicekcioglu H, Neselioglu S, Erel O, Ornek E. A novel oxidative stress marker in acute myocardial infarction; thiol/disulphide homeostasis. Am J Emerg Med. 2015;33:1567–1571.
Ladawan S, Klarod K, Philippe M, Menz V, Versen I, Gatterer H, Burtscher M. Effect of Qigong exercise on cognitive function, blood pressure and cardiorespiratory fitness in healthy middle-aged subjects. Complement Ther Med. 2017;33:39–45.
Lan C, Lai JS, Chen SY, Wong MK. Tai Chi Chuan to improve muscular strength and endurance in elderly individuals: a pilot study. Arch Phys Med Rehabil. 2000;81:604–607.
Larkey L, Jahnke R, Etnier J, Gonzalez J. Meditative movement as a category of exercise: implications for research. J Phys Act Health. 2009;6:230–238.
Li F, Harmer P, McAuley E, Duncan TE, Duncan SC, Chaumeton N, Fisher KJ. An evaluation of the effects of Tai Chi exercise on physical function among older persons: a randomized controlled trial. Ann Behav Med. 2001;23:139–146.
Mendoza-Nunez VM, Hernandez-Monjaraz B, Santiago-Osorio E, Betancourt-Rule JM, Ruiz-Ramos M. Tai Chi exercise increases SOD activity and total antioxidant status in saliva and is linked to an improvement of periodontal disease in the elderly. Oxid Med Cell Longev. 2014;2014:603853
Nielsen F, Mikkelsen BB, Nielsen JB, Andersen HR, Grandjean P. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43:1209–1214.
Oh DH, Park JE, Lee ES, Oh SW, Cho SI, Jang SN, Baik HW. Intensive exercise reduces the fear of additional falls in elderly people: findings from the Korea falls prevention study. Korean J Intern Med. 2012;27:417–425.
Pan XH, Mahemuti A, Zhang XH, Wang YP, Hu P, Jiang JB, Xiang MX, Liu G, Wang JA. Effect of Tai Chi exercise on blood lipid profiles: a meta-analysis of randomized controlled trials. J Zhejiang Univ Sci B. 2016;17:640–648.
Radak Z, Chung HY, Goto S. Exercise and hormesis: oxidative stress-related adaptation for successful aging. Biogerontology. 2005;6:71–75.
Reid MB, Khawli FA, Moody MR. Reactive oxygen in skeletal muscle. III. Contractility of unfatigued muscle. J Appl Physiol. 1993;75:1081–1087.
Robinson R. Taiji Qigong 18 movements [Internet]. Chiron Tai Chi; c2020. [cited 2017 Jun 29]. Available from http://www.chirontaichi.co.uk/downloads/taiji_qigong_18_movements.pdf
.
Rosado-Perez J, Ortiz R, Santiago-Osorio E, Mendoza-Nunez VM. Effect of Tai Chi versus walking on oxidative stress in Mexican older adults. Oxid Med Cell Longev. 2013;2013:298590
Sakata T, Li Q, Tanaka M, Tajima F. Positive effects of a qigong and aerobic exercise program on physical health in elderly Japanese women: an exploratory study. Environ Health Prev Med. 2008;13:162–168.
Sato Y, Nanri H, Ohta M, Kasai H, Ikeda M. Increase of human MTH1 and decrease of 8-hydroxydeoxyguanosine in leukocyte DNA by acute and chronic exercise in healthy male subjects. Biochem Biophys Res Commun. 2003;305:333–338.
Song QH, Zhang QH, Xu RM, Ma M, Zhao XP, Shen GQ, Guo YH, Wang Y. Effect of Tai-chi exercise on lower limb muscle strength, bone mineral density and balance function of elderly women. Int J Clin Exp Med. 2014;7:1569–1576.
Tebay LE, Robertson H, Durant ST, Vitale SR, Penning TM, Dinkova-Kostova AT, Hayes JD. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic Biol Med. 2015;88:108–146.
Thornton EW, Sykes KS, Tang WK. Health benefits of Tai Chi exercise: improved balance and blood pressure in middle aged women. Health Promot Int. 2004;19:33–38.
Tracy BL, Byrnes WC, Enoka RM. Strength training reduces force fluctuations during anisometric contractions of the quadriceps femoris muscles in old adults. J Appl Physiol. 2004;96:1530–1540.
Trajkovic N, Gusic M, Molnar S, Macak D, Madic DM, Bogataj S. Short-term FIFA 11+ improves agility and jump performance in young soccer players. Int J Environ Res Public Health. 2020;17:2017
Tremblay MS, Aubert S, Barnes JD, Saunders TJ, Carson V, Latimer-Cheung AE, Chastin SFM, Altenburg TM, Chinapaw MJM. SBRN Terminology Consensus Project Participants. Sedentary Behavior Research Network (SBRN) - Terminology Consensus Project process and outcome. Int J Behav Nutr Phys Act. 2017;14:75
Tsai LY, Lee KT, Tsai SM, Lee SC, Yu HS. Changes of lipid peroxide levels in blood and liver tissue of patients with obstructive jaundice. Clin Chim Acta. 1993;215:41–50.
Tsang HW, Mok CK, Au Yeung YT, Chan SY. The effect of Qigong on general and psychosocial health of elderly with chronic physical illnesses: a randomized clinical trial. Int J Geriatr Psychiatry. 2003;18:441–449.
Wittwer M, Billeter R, Hoppeler H, Fluck M. Regulatory gene expression in skeletal muscle of highly endurance-trained humans. Acta Physiol Scand. 2004;180:217–227.
Wolf SL, Barnhart HX, Kutner NG, McNeely E, Coogler C, Xu T. Reducing frailty and falls in older persons: an investigation of Tai Chi and computerized balance training. Atlanta FICSIT Group. Frailty and Injuries: Cooperative Studies of Intervention Techniques. J Am Geriatr Soc. 1996;44:489–497.
Fig. 3Correlations between changes (delta, difference POST minus PRE) in back strength and changes (delta, difference POST minus PRE) in oxidative stress (MDA) including both the Qigong and the control group. 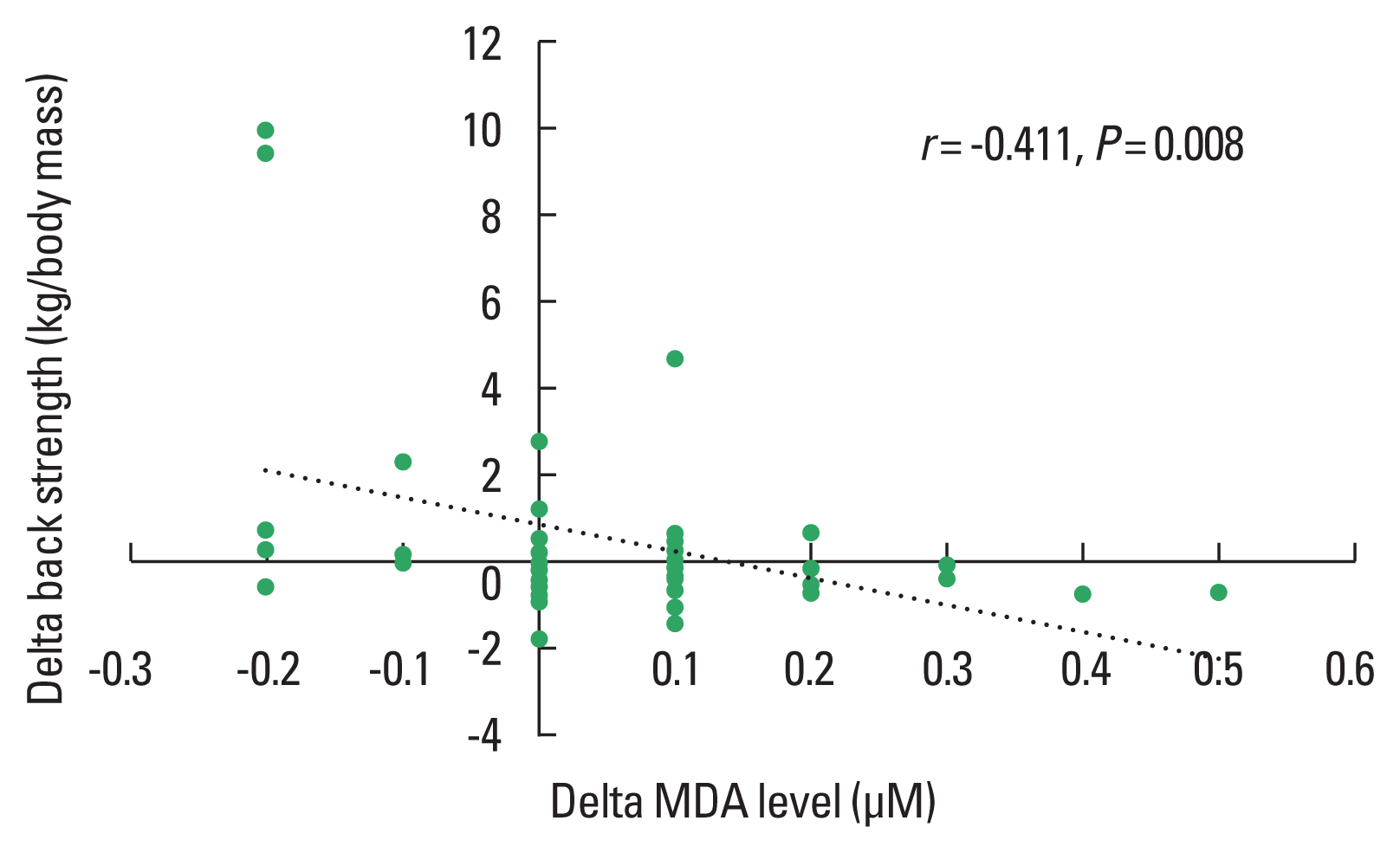
Fig. 4Correlations between changes (delta, difference POST minus PRE) in leg strength and changes (delta, difference POST minus PRE) in oxidative stress (MDA) including both the Qigong and the control group. 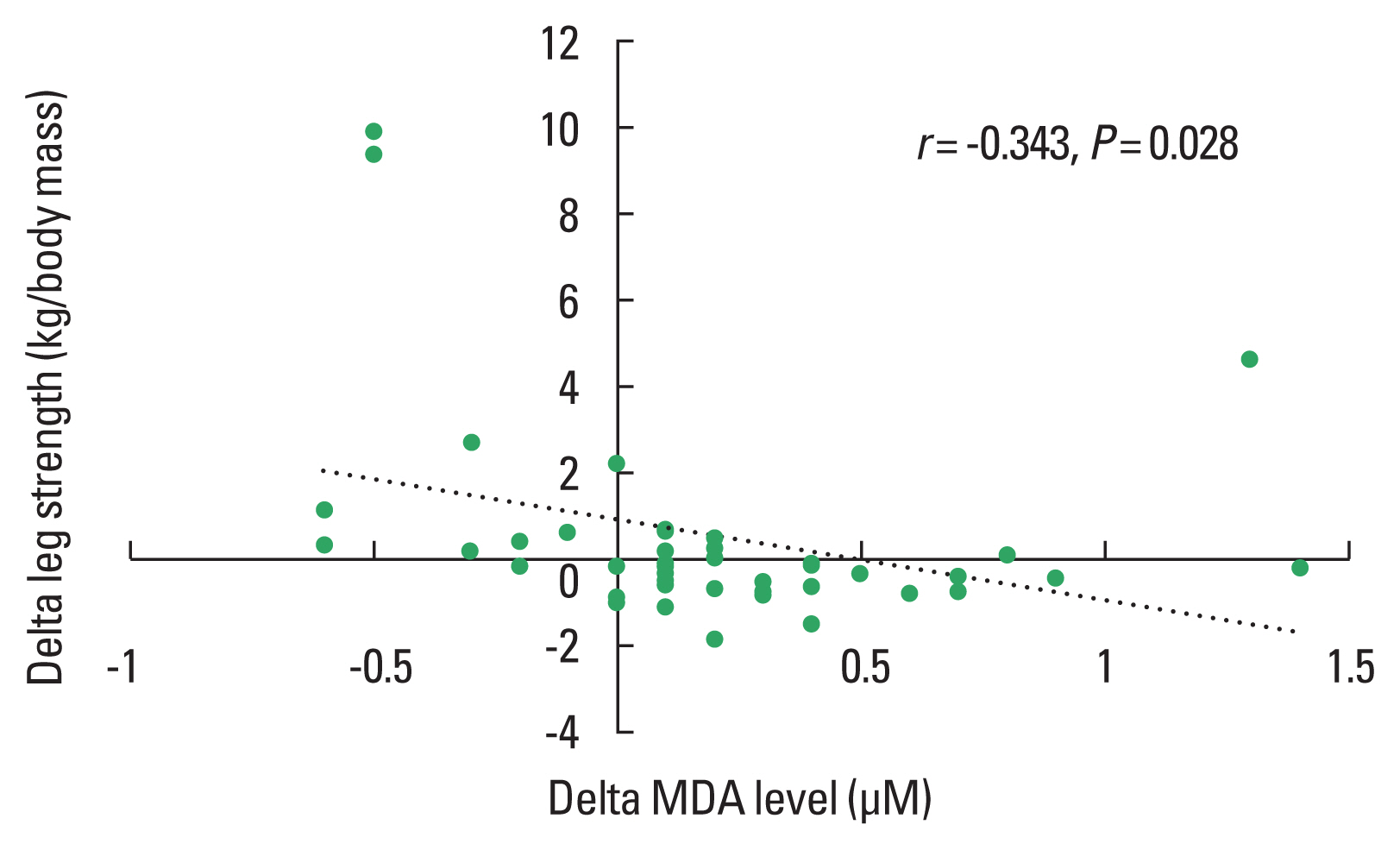
Fig. 5Correlations between changes (delta, difference POST minus PRE) in leg strength and changes (delta, difference POST minus PRE) in total antioxidant (TAC) levels including both the Qigong exercise group and the control group. 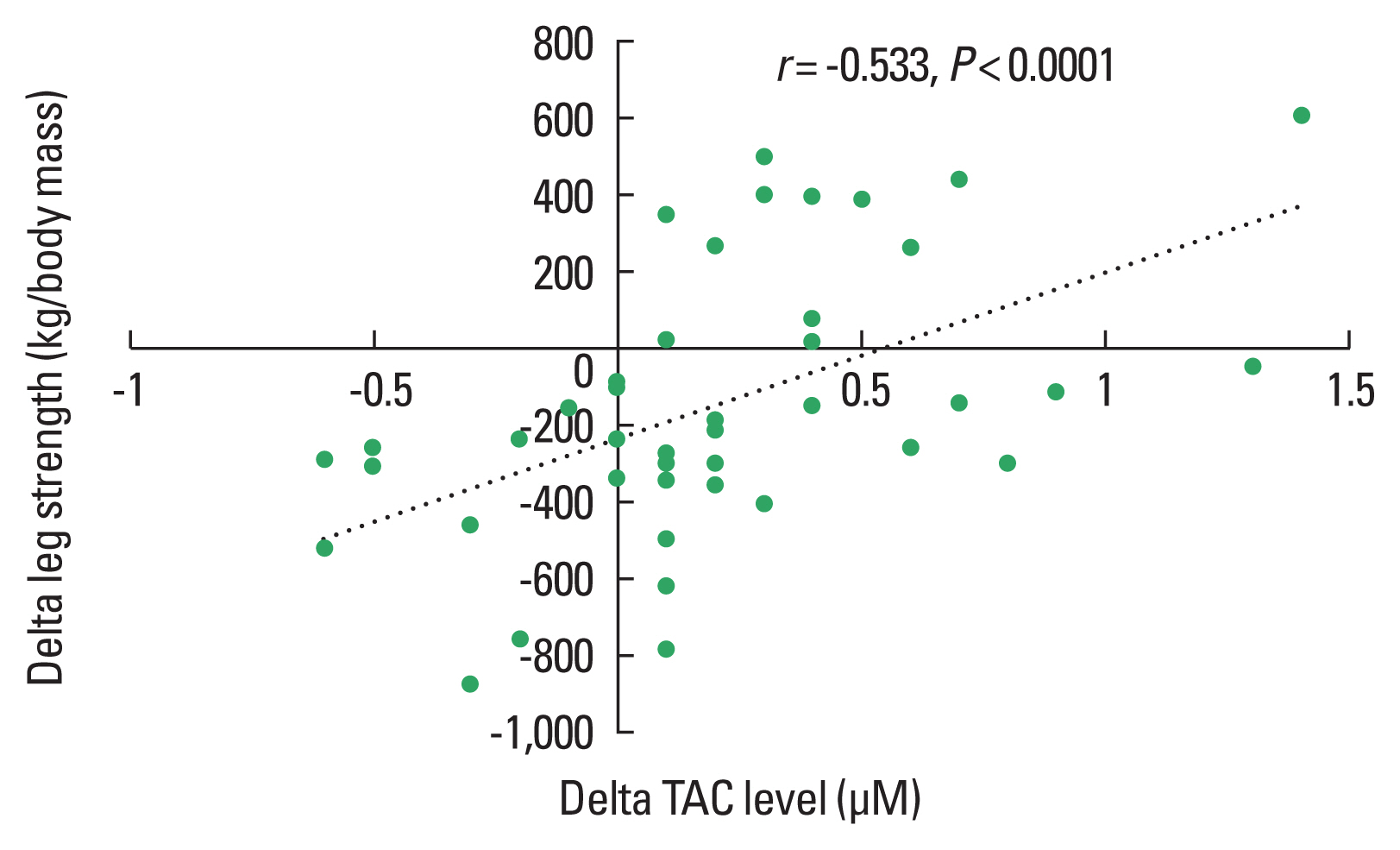
Table 1Baseline characteristics of the Qigong (QG) and the control group (CG)
Table 2Physical performances at baseline (PRE) and after the intervention (POST) in the Qigong (QG) and the control group (CG)
Table 3Oxidative stress, nonenzymatic and enzymatic antioxidants at baseline (PRE), after the first training session (ACUTE), and after the 8-week intervention period (POST) in the Qigong (QG) and the control group (CG)
|
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||