INTRODUCTION
Proprioceptive neuromuscular facilitation (PNF) works with the muscle and joint proprioceptors to facilitate neuromuscular system performance (Showman, 1962). PNF uses diagonal patterns of movement with maximal muscle strength, in isotonic and isometric muscle contractions, to achieve the desired objectives (Rhyu et al., 2015). To facilitate the contraction of the weak muscles, PNF uses force muscle irradiation, stimulating the strong muscles (Abreu et al., 2015; Gontijo et al., 2012; Hindle et al., 2012; Meningroni et al., 2009; Pink, 1981). Force muscle irradiation is the process where a stimulus reaches the spinal cord through the peripheral nervous system and then returns to the muscle (Ivanenko et al., 2004).
PNF is widely used for rehabilitation in patients with encephalic dysfunctions (Krukowska et al., 2016; Ribeiro et al., 2014), however there are very few studies in patients with peripheral nervous system injury (Meningroni et al., 2009).
Charcot-Marie-Tooth (CMT) is part of a heterogeneous group of clinical diseases and genetic disorders. CMT is a hereditary motor and sensory neuropathy that produces progressive muscle atrophy and weakness of the extremities, sensory loss and decreased or absent tendon reflexes (Reilly et al., 2011). This neuropathy can be classified into type 1 or 2, but both are very similar, from a clinical standpoint. Type 1A is the most common form of CMT (CMT-1A) and is the result of duplication on chromosome 17p11.2, with encoding myelin protein, which causes a decrease in conduction velocity of peripheral nerves and distal muscle strength (Krajewski et al., 2000). Muscle weakness affects various muscles in the lower limbs (LLs). One muscle with clinical importance, for the CMT-1A patients, is the tibialis anterior muscle (TAm). The TAm failure manifests clinically as “foot drop,” and it is found in a high percentage of people affected by the disease (Sackley et al., 2009). These changes in muscle strength and ankle biomechanics can interfere with walking and contribute to foot deformities in this population (Burns et al., 2005).
Physical therapists utilize many approaches for foot drop rehabilitation, and PNF is one of them. We reported previously that PNF patterns produce overflow in patients with CMT-1A in contralateral upper limb (UL) and LL (Meningroni et al., 2009). Since it is possible to control TAm failure in CMT-1A, using contralateral overflow, it is essential to investigate other FNP patterns, to understand if they can produce overflow in patients with demyelinating polyneuropathy. Thus, this study analyzed ipsilateral PNF and its effects on TAm, after five weeks of treatment.
MATERIALS AND METHODS
Participants
We evaluated and treated 13 individuals of both sexes, with CMT-1A disease, aged 15 to 64 years, presenting muscle weakness and abnormal gait. All patients were recruited from the outpatient clinic of Neurogenetics, Department of Neuroscience and Behavioral Sciences of the Hospital of Ribeirão Preto Medical School, at the University of São Paulo (Hospital das Clínicas, HCFMRP/USP). The CMT-1A diagnosis was given by a neurologist, using family history, as well as electrophysiological and genetic testing. The subjects were in different stages of the disease, ranging from moderate to severe.
The inclusion criteria were: (a) preserved cognition, (b) no joint blockages (range of motion preserved), and (c) ability to walk, with or without assistance. The exclusion criteria were: (a) cardiac arrhythmias, (b) uncontrolled hypertension, and (c) severe cardiovascular and respiratory problems. The use of medications for other diseases was accepted. All participants signed an informed consent form, prior to the start of the study.
The Ethics in Human Research Committee of HCFMRP/USP (protocol n° 10530/2007) approved this project. This methodology was registered in the National System of Information Ethics and Human Research (CAAE- 0445.0.004.000-07) to be appropriate, ethically and methodologically, as per the precepts of Resolution 196/96 of the National Health Council.
Study protocol
The participants were assigned to one single treatment group (single-subject design study). The PNF patterns adopted in this study aimed to produce ipsilateral overflow in the TAm. Each patient’s evaluation happened before the first and after the last treatment session (Fig. 1).
Physical therapy sessions using PNF were performed twice per week, for a total of five weeks and ten therapy sessions. The International Association of Proprioceptive Neuromuscular Facilitation certified the physiotherapist who was responsible for the PNF sessions. We use the following PNF patterns:
Chopping: The patients were seating on the stretcher with both shoulders in flexion, abduction and external rotation; elbows in extension and wrist and fingers in flexion. The patients shook the physical therapist’s hand; and moved both UL simultaneously in the direction of the hip at left or at right, without flexing the elbow (Showman, 1962).
Flexion-abduction-internal rotation (LLD1): The patients were positioned in the supine position, lying down on the stretcher. One of the LL was positioned in extension, external rotation, and adduction. They were instructed to perform flexion, abduction, and internal rotation (Showman, 1962).
Extension-adduction-external rotation (LLD2): The patients were positioned in the supine position, lying down on the stretcher. One of the LL was positioned in flexion, abduction and internal rotation of the hip. They were instructed to perform extension, adduction and external rotation (Showman, 1962).
All patterns of movement met resistance from the physical therapist’s hands. The maximum resistance was used, that is, the resistance that was tolerated by each patient, and that allowed for the execution of the movement pattern in a complete range of motion. All patterns were repeated four times, with an average execution time of 6 sec. The patients individually determined the resting period between the patterns. Each session lasted an average of 40 min. During the treatment, we respected the functionality, strength and muscular endurance of each patient. The same physical therapist applied all the patterns. Evaluations and assistance were provided at the Rehabilitation Center– HCFMRP/USP.
Neurophysiologic measurement: electromyography
Electromyography was performed by a 4-channel electromyography biofeedback equipment EMG-610C model, with a band-pass filter and a cutoff frequency between 20–500 Hz, amplified with a 1,000 gain and common mode bounce rate >120 dB (EMG System of Brazil, São José dos Campos, Brazil). We used a notebook HP Pavillion dv2000 brand, with AMD Turion 64X2. The equipment was connected to the battery, with no contact with the electrical network for stabilizing the electromyography (EMG) signal and filtering possible noises from the power supply.
A disposable surface electrode, double and bipolar Ag/AgCl (silver/silver chloride), made with polyethylene hypoallergenic foam medical adhesive, was placed bilaterally on the TAm muscle belly, with a fixed interelectrode distance of 20 mm. We used a disposable razor to remove the hair from the skin. An abrasion was performed using a nail file to remove dead skin cells, and the area was subsequently cleaned with 70% alcohol (Costa and de Araujo, 2008; de Souza et al., 2016; Zanin et al., 2014).
The EMG signal in root mean square (RMS) was obtained during the execution of the PNF patterns. Since each pattern had an average duration of 6 sec, we discarded the first and final seconds of each sampling. For data acquisition, DATAQ Instruments Hardware Manager software (DATAQ Instruments, Akron, OH, USA) was used. All procedures mentioned above are in accordance with the recommendations of the European Union for surface electromyography noninvasive muscular project (SENIAM project, 2006).
All subjects performed active dorsiflexion at the beginning of the first session, with the right and left foot. The physical therapists verbally requested that the patient use the highest strength possible to perform the movement.
Statistics
The patterns were repeated 4 times for each side of the body, thus, we used the average of the four RMS values for each pattern ([RMS execution1+RMS execution 2+RMS execution 3+RMS execution 4]÷4=RMS pattern). RMS data obtained in the first session were normalized and transformed into the percentage. Therefore, the average RMS for each diagonal, at the beginning of the treatment, corresponds to 100% of TAm activation. RMS data obtained in the last session were normalized as the percentage, taking into account the results of the first evaluation. The data (first and last session) showed variance and normality according to the Kolmogorov–Smirnov test, thus it was analyzed by Student paired t-test, the P-value was set at <0.05. The clinical significance was obtained by the subtraction of the values obtained in the last session by the values obtained in the first session. This data was presented in percentage.
RESULTS
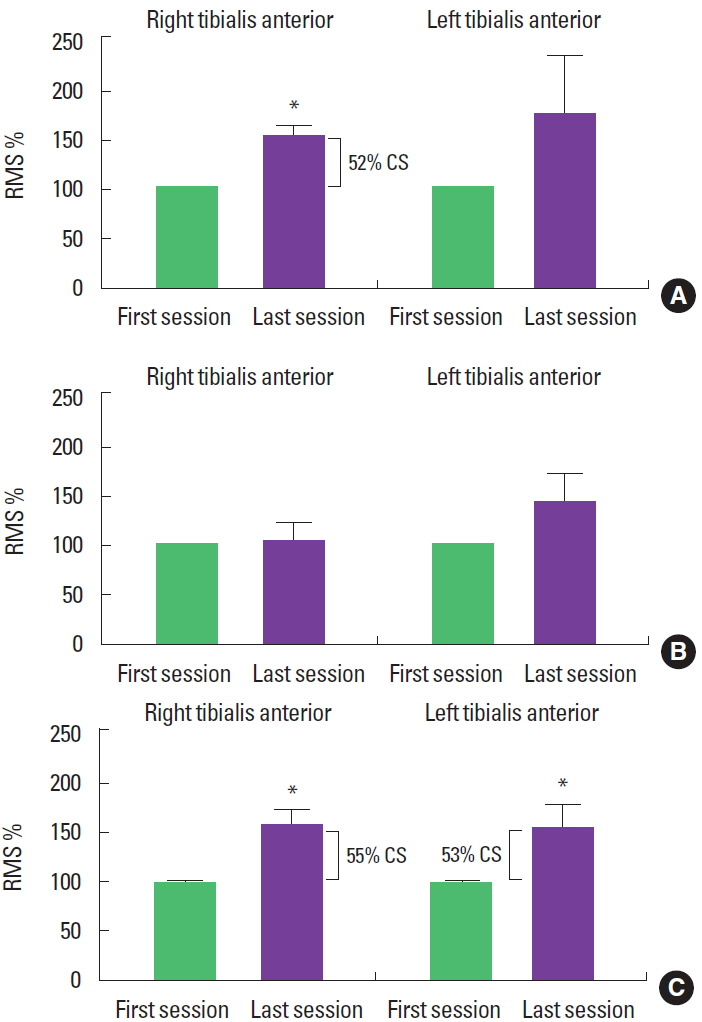
Ipsilateral force overflow was produced by chopping pattern on the right side of the body, as RMS values in the TAm increased, when comparing the last session to the first (t=−3.52, P<0.05). However, the same was not true for the patterns applied to the left side of the body (t=−3.35, P=0:21). In the LLD2 pattern, we did not find any changes in the TAm RMS values, for right or left (t= −2.46, P=0.80 and t=−1.84, P=0:10, respectively). On the other hand, the ipsilateral LLD1 pattern produced overflow, as an increase in the TAm RMS values on the right and left sides (t= −2.46, P<0.05 and t=−2.07, P<0.05, respectively). Clinically, overflow obtained with the chopping pattern on the right was 52%, and the LLD1 pattern was 55% for the right and 53% for the left side (Fig. 2).
DISCUSSION
The patients who participated in our study showed the inherent muscle weakness for TAm activation that is characteristic of the CMT-IA disease. This weakness is caused by the myelin sheath modification, which reduces the conduction speed on the peripheral nerves (Borg and Ericson-Gripenstedt, 2002). Despite the reduced speed conduction of the stimulus in the peripheral nerves, our results show that a short-term intervention program, based on the ipsilateral pattern of PNF, can improve the TAm RMS response.
Rehabilitation treatments can offer possibilities to improve the muscle strength in CMT-1A patients. The progressive resistance-training program applied directly to the weak muscles (Chetlin et al., 2004) and the moderate intensity muscle training (Kilmer, 2002) seems to be adequate for these patients. In general, the effectiveness of these techniques can be attributed to an exercise of low duration that takes into account the individual response and the stage of the disease. This corroborates the well-known fact that high-intensity exercises do not bring improvements to this population (Fowler, 2002).
Our PNF protocol used a short duration muscle contraction, with low repetition of patterns and longer resting time, as described by other authors (Chetlin et al., 2004; Kilmer, 2002). The patients can perform the patterns using the full range of motion and, despite the maximal muscle resistance, our protocol is characterized as moderate exercise. Furthermore, the exercises are performed by the strong muscle groups, and not by the weak muscle groups, that need to be treated, and this is highly relevant because it allows the patients to actively participate in the treatment.
Resistance exercises, in healthy subjects, can produce recruitment of other muscle groups as the synergists, improving the movement strength and producing contralateral overflow (Pink, 1981). Additionally, previous work from our research group showed that PNF contralateral patterns in CMT-1A patients increase the TAm activation (Meningroni et al., 2009). While studies with overflow show this effect in the contralateral side of the body, our results show the possibility of producing overflow on the same side of the body (Abreu et al., 2015; Gontijo et al., 2012).
Studies in the literature have consistently shown that older adults use homologous muscles contralaterally, during voluntary contractions (Bodwell et al., 2003; Shinohara et al., 2003). This involuntary muscle response changes the corticospinal output (Gross et al., 2005) and modulates the corticomuscular coherence (Johnson et al., 2011). This concept is fundamental to our results, because if CMT-1A does not allow for peripheral nerve improvement, then the effect that we showed in the present study is related to a central nervous system adaptation. It is known that neuromuscular adaptation occurs from resistance exercises, which changes the way the muscles are recruited by the central nervous system, allowing for motor learning and better muscle recruitment (Carroll et al., 2001).
Despite the possible corticospinal effect, the PNF TAm activation seen here is likely related to spinal circuits involved with control of rhythmic locomotion (Guertin, 2013). While flexion of the trunk, hip, and knee produced flexion of the hip; LL extension produces ankle extension that facilitates plantar flexion movement. This is most likely why chopping and LLD1 improved the TAm activation, while LLD2 did not. Thus, it is extremely important to understand the biomechanical pattern of body movement for producing a substantial overflow.
The present results are consistent with the results from our previous study (Meningroni et al., 2009) using PNF contralateral patterns which activated TAm in CMT-1A. Our results show that the ipsilateral patterns of FNP can be a reasonable and helpful treatment for foot disorders in this population. The TAm effect is present while the treatment is in progress.
The present study is limited by the lack of follow up after the treatment due to the need for travelling far to the HCFMRP/USP Hospital to participate. Thus, there is need for further studies, to follow these patients for a more extended period, to see if the TAm effects remain.