INTRODUCTION
As an endocrine organ, white adipose tissue (WAT) regulates systemic energy homeostasis. Since WAT has a strong plasticity, its expansion according to overnutrition is associated with metabolic diseases. WAT expansion is highly depends on angiogenesis that is defined as a formation of new blood vessels from pre-existing vasculature. When adipose tissues are expanded, adipogenesis is followed by the generation of dense vascular network (DVN) in rodents and introduction of antiangiogenic agents such as soluble vascular endothelial growth factor receptor 2 (Flk-1) or vascular endothelial growth factor A (VEGFA) inhibitor prevents the formation of DVN, resulting in the enhancement of antiadipogenic effects (Cho et al., 2007). Additionally, an angiogenic inhibitor TNP-470 treated ob/ob mice reduced body weight gain by diminishing adiposity (Rupnick et al., 2002), supporting that angiogenesis is accompanied with adipogenesis.
Angiogenesis is induced by angiogenic factors and major angiogenic factors expressed from WAT include VEGF, angiopoietin (Ang) and platelet-derived growth factor (PDGF). VEGF family has VEGFA, VEGFB, VEGFC, and VEGFD. VEGFA, the predominant form, has five different isoforms (VEGF121,VEGF165, VEGF189, and VEGF206) and binds to its receptor VEGFR1 (Flt-1) and VEGFR2 (Flk-1). Since VEGFA induced Flk-1 activation is the key regulator of endothelial cell survival and growth (Ferrara et al., 2003), VEGFA haplodeficient mouse shows a lethal phenotype in embryonic days 11–12 due to impairment of blood vessel development and deficiency of red blood cell in blood island of york sac (Carmeliet et al., 1996; Ferrara et al., 1996). In addition, VEGFA deletion in perineonatal mouse using cre-loxP system brings the defect of tissue and vascular developments (Gerber et al., 1999). Similar with VEGFA deficient phenotype, VEGFR2 deficient mouse also fails to vascular development by impairing differentiation of endothelia cell and hematopoietic stem cell (Shalaby et al., 1995). Ang family has Ang1, Ang2, and Ang3. While Ang1 is expressed on smooth muscle cells, pericytes and fibroblast, Ang2 is almost exclusively expressed from endothelial cells. Both Ang1 and Ang2 bind to their receptor, TEK tyrosine kinase receptor 2 (Tie2) that is expressed on endothelial cell and macrophagy (Fagiani and Christofori, 2013). As the major function of Ang1 is to regulate vascular remodeling and maturation, Ang1 or Tie2 deletion mice are lethal (Suri et al., 1996). Moreover, Ang1 enhances integrity of vasculature by recruiting pericytes into blood vessel, therefore, Ang1 overexpression mouse increases endothelial cell quiescent (Cho et al., 2005; Suri et al., 1998). Also, exogenous Ang1 treatment is beneficial for the recovery from injured tissues by inducing angiogenesis (Chae et al., 2000; Jung et al., 2009; Park et al., 2009; Shin et al., 2010; Smith et al., 2012). In contrast to Ang1, Ang2 acts as an antagonist of Ang1. Ang2 is able to bind integrin and then prevents anti-inflammatory function of Ang1 (Felcht et al., 2012; Gale et al., 2002). In the same context, Ang2 overexpression brings embryonic lethality by inhibiting vascular development, which is shown in Ang1 or Tie2 deficient mouse (Maisonpierre et al., 1997; Suri et al., 1996). Interestingly, plasma Ang2 concentration is upregulated in inflammation and Type2 diabetes (Fiedler et al., 2006; Li et al., 2015), meaning that inflammation might be a key regulator of Ang2 expression.
PDGF family is composed of five dimeric isoforms, PDGF-AA, PDGF-AB, PDGF-BB, PDGF-CC, and PDGF-DD. PDGF family bind to PDGF receptor (PDGFR)α or PDGFRβ. Specifically, PDGF-BB that is important stimulus for the differentiation of embryonic stem cell into endothelial cell in developmental stage and it regulates proliferation, migration and tube formation of endothelial cell (Lange et al., 2009). In adults, PDGF-BB that is mainly expressed from endothelial cell is involved in the recruitment of PDGFRβ+ pericytes and enhances blood vessel stability (Levéen et al., 1994; Soriano, 1994).
Angiogenesis in adipose tissue is mediated by angiogenic factors. Although adipose tissue specific VEGFA deleted mouse reduces fat mass under high fat diet condition, it causes insulin resistance since lipid designated to adipose tissue is accumulated in other tissues such as liver and skeletal muscle. In the opposite way, adipose tissue specific overexpression of VEGFA increases insulin sensitivity by diminishing local hypoxia in adipose tissue (Sung et al., 2013). Ang1 and Ang2 expressions are also altered depending on the augmentation of adipose tissues (An et al., 2017; Dallabrida et al., 2003). In addition, whole body PDGFRβ deficiency improves adipocity and insulin sensitivity in high fat diet induced obese mouse (Onogi et al., 2017).
Exercise has been shown to modulate angiogenic gene expression in heart and skeletal muscle (Erekat et al., 2014; Gustafsson et al., 1999; Lloyd et al., 2003; Trenerry et al., 2011). However, it has not been extensively investigated whether exercise regulates angiogenic gene expression in adipose tissue. To interrogate the effect of exercise training on angiogenic gene expression, mice were performed voluntary wheel for 6 weeks and then the mRNA levels of VEGFA, Angs, PDGF-B and their receptors were analyzed in this study.
MATERIALS AND METHODS
Mice
Male C57BL/6J mice at 5 weeks of age were purchased from Central Lab. Animal Inc. (Seoul, Korea). Mice were housed in an environmentally controlled facility certified by the Office of Laboratory Animal Welfare with a 12-hr light/dark cycle and had free access to food and water.
All animal experiments were proved by The Institutional Animal Care and Use Committee of Dongguk University (IACUC-2017-017).
Exercise protocol
After 2 weeks of acclimation, C57BL/6J male mice at 7 weeks of age were randomly divided into two groups: a sedentary control group and a voluntary wheel running exercise group (n=8 in each group). Mice in exercise group were housed individually in wheel cage (Nalgene) where mice are able to run voluntarily on the wheel all the time. The wheel revolution was automatically counted and the average of running distance for each mouse was calculated at the end of 6 weeks. The average distance of exercise group was 4.96±2.77 km/day. Age and sex matched control mice were housed individually in cage without wheel. After 6 weeks of training, mice were sacrificed 24 hr after the last exercise training.
Tissue isolation
For the analysis of angiogenic gene expression, tibialis anterior (TA), soleus (Sol), epididymal WAT (eWAT), inguinal WAT (iWAT), and brown adipose tissue (BAT) from mice in control and exercise groups were isolated and then were snap frozen in liquid nitrogen and stored at −80°C for further analysis.
RT-PCR
Total RNA was extracted from TA, Sol, eWAT, iWAT, and BAT collected from control and exercise groups using TRIzol isolation reagent (Invitrogen, Carlsbad, CA, USA) under the manufacturer’s instructions. RNA concentration was spectrophotometrically determined using NanoDrop (Thermo Fisher Scientific, Waltham, MA, USA). Using murine leukaemia virus reverse transcriptase and oligo (dT)16 primer, two micrograms of tissue RNAs were reverse transcribed. The resulting cDNAs from tissue samples were assayed in duplicate. Reverse transcription-polymerase chain reaction (RT-PCR) was conducted using 2X SYBR green PCR master mix on a real-time PCR system (Applied Biosystems, Waltham, MA, USA). Gene expression data were normalized to the housekeeping gene GAPDH and analyzed using the delta delta cycle threshold method (ΔΔCt). Primer sets are described in Table 1.
RESULTS
Exercise training reduces WAT mass
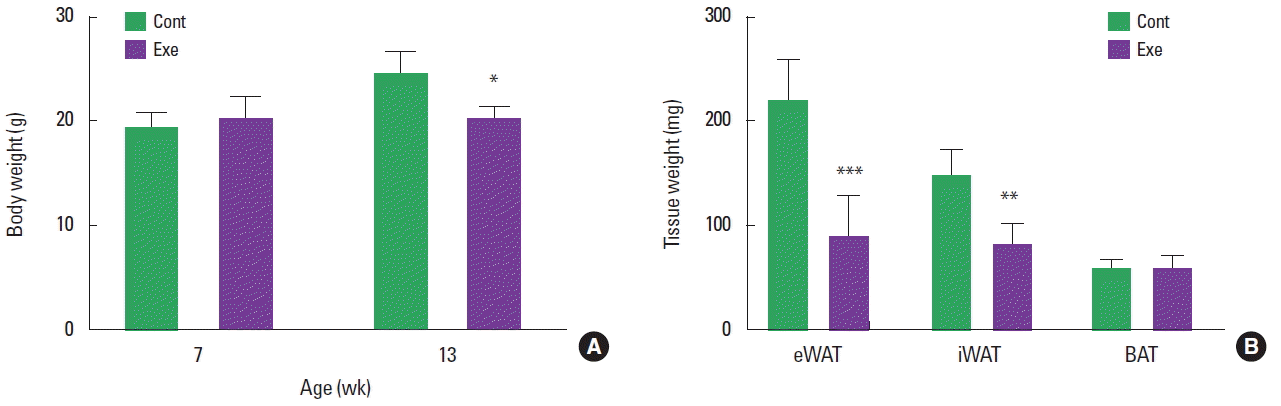
To identify the change of body weight and adipose tissue weight by exercise training, body weight were measured before and after exercise training and adipose tissue weight were measured after exercise training. After 6 weeks of voluntary wheel running exercise, the body weight was decreased only in exercise group (Fig. 1A). And the fat weight was reduced 40% in eWAT (220.90±39.32 mg vs. 87.32±40.53 mg, P<0.001) and iWAT (146.85±26.45 mg vs. 78.74±23.02 mg, P<0.01) respectively after 6 weeks of training. However, BAT weight was comparable between groups (57.30±8.92 mg vs. 55.70±14.60 mg, P>0.05).
VEGFA expression is reduced in WAT after exercise training
Since VEGFA is one of the strongest angiogenic factors, its expression was analyzed in TA, Sol, eWAT, iWAT, and BAT with RT-PCR. Interestingly, the expression level of VEGFA in Sed was higher in eWAT (13 fold) and iWAT (3 fold) than that of skeletal muscle or BAT. VEGFA expression was significantly increased in both of skeletal muscles and iWAT after 6 weeks of exercise training. Unlike skeletal muscle and iWAT, VEGFA expression was dramatically decreased in eWAT after training (Fig. 2A). Although the induction of VEGF in skeletal muscle was increased about 2 fold after exercise training, Flk-1 expression was not changed. Instead, exercise training dramatically decreased the expression of Flk-1 in eWAT (Fig. 2B).
Exercise training regulates Ang expression in WAT
Angiopoietin tightly regulates angiogenesis and vascular integrity. The expression level of Ang1 in control group was relatively higher in eWAT (2 fold) and iWAT (3 fold) than that of skeletal muscle or BAT, whereas Ang2 expression in control group was 2 times higher in iWAT than those of other tissues (Fig. 3A, B). After 6 weeks of exercise training, Ang1 expression was significantly reduced in only iWAT (Fig. 3A). On the other hands, Ang2 expression was significantly induced in eWAT after exercise training (Fig. 3B). However, Tie2 expression was not altered after exercise training (Fig. 3C).
PDGFRβ expressions are altered in eWAT after exercise training
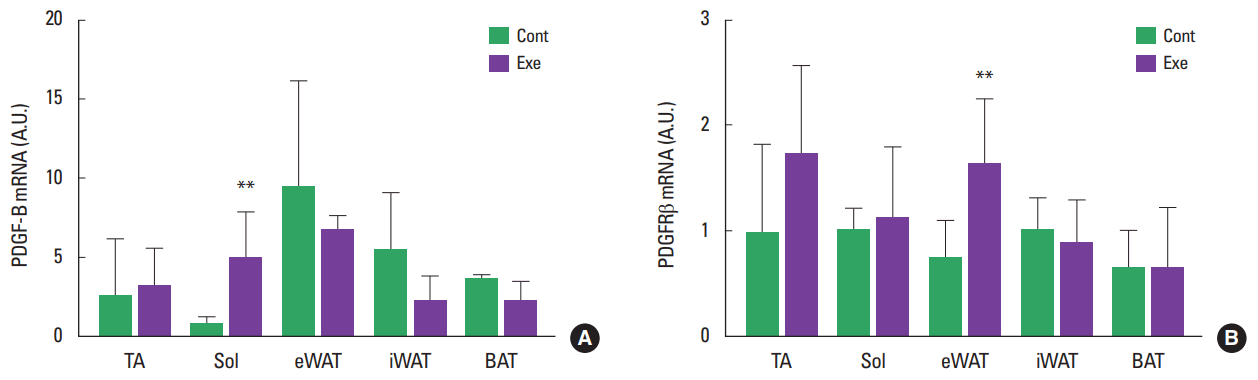
To determine whether exercise training regulated the expression of PDGF-B and its receptor PDGFRβ, mRNA expression was analyzed with skeletal muscles and adipose tissues after 6 weeks of voluntary wheel running. Similar with the expression levels of VEGFA and Ang1, PDGF-B expression was higher in eWAT (3.6 fold) and iWAT (2.5 fold) than TA in control group (Fig. 4A), however there was no alteration of PDGF-B in adipose tissues after exercise training. Instead, the expression of PDGF-B was significantly increased in Sol after exercise training (Fig. 4A), but the expression level of PDGFR-β was not changed in Sol after exercise training. Instead, its expression was increased in eWAT after exercise training (Fig. 4B).
DISCUSSION
It is well known that exercise training regulates angiogenesis by stimulating angiogenic gene expression in heart and skeletal muscle (Erekat et al., 2014; Gustafsson et al., 1999; Lloyd et al., 2003; Trenerry et al., 2011). Besides heart and skeletal muscle, VEGFA-induced vascular remodeling plays a key role in adipose tissue function in systemic glucose homeostasis that impacts on insulin resistance (Sung et al., 2013). Adipose tissue responses differently to energy intake or expenditure and its mass is reduced by exercise training since fatty acid from adipose tissue is utilized during exercise as fuel (Horowitz, 2003). However, it has not been clear whether angiogenic gene expression is altered in reduced adipose tissue after exercise training. To identify the expression level of angiogenic genes after exercise training, mice were performed voluntary wheel running for 6 weeks. Although treadmill or swimming exercise that generates reproducible speeds or distances is regarded as a standardized exercise protocol for rodents, electrical stimulus or environmental stress on mouse for the force active movement might jeopardize the effects of exercise. Therefore, voluntary wheel running exercise was applied to avoid of additional stresses except exercise in the current study.
Previous studies showed that exercise training with voluntary wheel reduced body weight and adiposity in diet-induced obese mice as well as normal diet treated lean mice (Reynolds et al., 2015). Consistently, body weight was significantly decreased with the reduction of WAT weights in Exe after 6 weeks of voluntary wheel running in this study (Fig. 1). The reduction of adipose tissue mass after exercise is attributed to the hypotrophy of adipocytes. Hypotrophy of white adipocytes in obese individuals after exercise training results from lipolysis regulated by hormone sensitive lipase and adipose triglyceride lipase (Ogasawara et al., 2010; Ogasawara et al., 2012). After confirming that the weights of eWAT and iWAT were reduced in exercise group (Fig. 1B), angiogenic gene expression was analyzed.
Exercise training increased VEGFA expression in heart and skeletal muscle. Swimming exercise for 15 days increased cardiac vascularization and swimming exercise combined VEGF treatment accelerated angiogenic process in heart (Efthimiadou et al., 2004). Indeed, both single exercise and endurance training upregulated VEGFA expression in skeletal muscle (Breen et al., 1996; Lloyd et al., 2003). However, upregulated VEGF was downregulated to normal level after exercise training (Lloyd et al., 2003). Although, increased fat mass showed positive correlation with VEGFA expression (Miyazawa-Hoshimoto et al., 2005), it has not been intensively studied whether VEGFA expression is altered in downsized adipose tissue after exercise training. After 6 weeks of voluntary wheel running exercise in this study, VEGFA expression was upregulated in TA and Sol as described previously, whereas its expression was dramatically downregulated in eWAT. Unlike eWAT, VEGFA expression was increased in iWAT despite the reduction of its mass after exercise training (Fig. 2A). VEGF is expressed in mature adipocytes of rodents and its expression is higher in visceral fat than subcutaneous fat (Fain et al., 2004; Miyazawa-Hoshimoto et al., 2005). Furthermore, VEGFA secretion from 3T3L-1 cells (a cell line cell of preadipocytes) kept being increased on the way of differentiation, indicating that lipid accumulation in adipocytes is related with VEGFA translation (Miyazawa-Hoshimoto et al., 2005). Therefore, the downregulation of VEGFA expression in eWAT after exercise training might be caused by the reduction of adipocyte size in eWAT. At least in myocytes, VEGFA expression is mediated by peroxisome proliferator-activated receptor-γ coactivator 1 alpha (Chinsomboon et al., 2009), however, it has not been identified the key regulator of VEGFA expression in adipose tissue after exercise. Exercise also suppressed the expression of Flk-1, VEGFA receptor. Since endothelial cell survival and growth were tightly regulated by Flk-1 (Ferrara et al., 2003), downregulation of both VEGFA and Flk-1 in eWAT after exercise might be a physiologic response to help regression of blood vessel depending on the reduction of adipose tissue mass. Therefore, regulators of VEGFA and the density or number of blood vessel in adipose tissue after exercise training should be analyzed to explain the physiological meaning of VEGFA and Flk-1 downregulations in future studies.
In iWAT, Ang1, instead of VEGFA, was downregulated after exercise training. Ang1 is responsible for the vascular maturation and integrity by recruiting pericytes and vascular remodeling by enhancing angiogenesis via Tie2 receptor (Chae et al., 2000; Cho et al., 2005; Jung et al., 2009; Park et al., 2009; Shin et al., 2010; Smith et al., 2012; Suri et al., 1998). Compared with VEGFA, the function of Ang1 in adipose tissue is not clear. Differentiated 3T3L-1 cells expressed Ang1 and TNP-470 treatment in ob/ob mouse reduced adipose tissue mass accompanied with downregulation of Ang1 expression (Dallabrida et al., 2003). It indicates that Ang1 expression in adipocytes diminished by reduction of fat mass. Therefore, the downregulation of Ang1 in iWAT after exercise training might be caused by the reduction of fat mass and it might be related with the regression of blood vessel. But it is not still clear why Ang1 expression was altered only in iWAT, not eWAT. Although VEGFA and Ang1 are all important for the generation of new blood vessel, they might contribute to fat depot specifically for the regression of blood vessel upon the need of fat mass reduction. For the confirmation of fat depot specific function of Ang1 and VEGFA on vessel regression, the vasculature of adipose tissues after exercise training should be investigated with adipocyte specific VEGFA or Ang1 deficient mouse in future. On the other hand, Ang2 was upregulated in eWAT after exercise training. Although inflammation is known to be a key regulator of Ang2 that is an antagonist of Ang1 (Felcht et al., 2012; Fiedler et al., 2006; Gale et al., 2002; Li et al., 2015), it was recently reported that Ang2 had a pro-angiogenic function in adipose tissues. Adipose tissue specific overexpression of Ang2 using adiponectin promoter increased vascularization with induction of VEGFA during WAT expansion induced by high fat diet treatment. Additionally, Ang2 reduced tumor necrosis factor alpha expression in WAT under high fat diet treatment, indicating that Ang2 improved angiogenesis and inflammation in obese WAT (An et al., 2017). Therefore, Ang2 upregulation in eWAT after exercise might be a compensatory response for the reduction of VEGFA to maintain a certain number of blood vessels. Also VEGF induction in iWAT might results from the induction of Ang1 for compensation. To confirm the compensatory response in WATs, it is necessary to analyze of the alteration of these gene expressions from adipose tissue specific Ang1 and VEGFA deficient mice in future studies.
PDGF-B expression was increased in Sol and this finding is consistent with previous study (Trenerry et al., 2011) but its expression was comparable in adipose tissues. Instead, PDGFRβ expression was significantly upregulated in eWAT after exercise training. PDGFR-β is generally expressed on smooth muscle cells and pericytes (Levéen et al., 1994; Soriano, 1994). At least in adipose tissue, PDGFRβ+ pericytes are regarded as one of adipocyte precursor cells because they are able to turn into mature adipocytes (Tang et al., 2008). Recently, the function of PDGFR-β in adipose tissue was uncovered. PDGFR-β knock-out mouse attenuated pericytes detachment in WAT with decrease in adipocytes size and enhanced glucose metabolism and energy expenditure (Onogi et al., 2017). After 6 weeks of exercise, there was no change in PDGF-B expression in adipose tissues and it indicates that PDGFR-β signaling is not activated after exercise training. Exercise training suppresses the differentiation of stromal vascular fraction of adipose tissue into mature adipocytes (Sakurai et al., 2010). Therefore, upregulation of PDGFRβ after exercise might reflex the accumulation of PDGFRβ+ pericytes only in downsized eWAT that highly depends on adipocytes hyperplasia for expansion, not iWAT that expands its mass through adipocyte hypertrophy (Wang et al., 2013).
Collectively, exercise training with voluntary wheel running altered angiogenic gene expression in downsized WATs. For further studies of its physiological meaning and biological mechanism, the origin of VEGFA, Angs, PDGF-B and their receptors should be analyzed in adipose tissue with reporter mice and orchestration of angiogenic factors after exercise training need to be studies with adipose specific knock-out mouse in near future.