AbstractAcute high-intensity physical exercise is known to improve cognitive performance of children, including those with attention-deficit/hyperactivity disorder (ADHD). In this work, we investigated the acute effect of an aerobic stretching and moderate-intensity, health and happiness improving movement (HHIM) exercise on the cortical activity of children with and without ADHD using electroencephalography (EEG). Children aged 12 to 14 yr with combined-type ADHD and age-matched healthy controls participated in the study, performing two individual movements (n=79, 35 controls) and a single exercise bout (n=45, 18 controls). electroencephalographic signals were recorded before and immediately after each movement, and before and after acute exercise under resting conditions, to obtain absolute and relative power estimates for the theta (3.5–8 Hz), alpha (8–12 Hz), sensory motor rhythm (12–16 Hz), and beta (16–25 Hz) bands. After acute HHIM exercise, all children showed significant changes in their relative EEG, mainly in the theta and alpha bands. Individual movements were found to influence relative theta, alpha and beta, and theta-to-beta ratios. He presents aerobic stretching HHIM exercise has demonstrated acute effect on the cortical activity of children.
INTRODUCTIONAttention-deficit/hyperactivity disorder (ADHD) is one of the most common chronic childhood neurobehavioral disorders that often persist into adulthood (Barbaresi et al., 2013). The rates of ADHD diagnosis are increasing in most countries, resulting in growing consumption of stimulant drugs (Singh, 2008). However, the ADHD diagnosis is based on pathological symptoms that are difficult to distinguish from normal childhood behaviors. For this reason, the increase in school-age children diagnosed with ADHD and taking the medications may not correctly represent the actual increase in ADHD prevalence but rather reflect possible overdiagnosis (Singh, 2008; Volkow et al., 2003). Although stimulant drugs are considered relatively safe and effective for treating ADHD symptoms (Biederman and Faraone, 2005), potential adverse effects and the unknown safety of their long-term use may still pose risks for overuse (Hazell, 2011; U.S. Food and Drug Administration, 2013). The treatment efficacy is rarely maintained beyond the active intervention period, requiring continued treatment throughout the lifetime (Halperin et al., 2011). For the partial or nonresponders other treatment options are needed (Loo et al., 1999).
Deficiencies in the cognitive function of ADHD children have been attributed to dysfunction of prefrontal cortical networks associated with neurochemical dysregulation (Bush, 2011; Cubillo et al., 2011; Seidman et al., 2005). Animal studies have shown that physical exercise increases cerebral blood flow and promotes neuroplasticity in the hippocampus and dopamine circuits (Foley and Fleshner, 2008; Swain et al., 2003; Van Praag et al., 1999). This provides a basis for investigating physical exercise as a safe and lasting treatment for children with ADHD. However, while the beneficial effects of acute physical exercise have focused on high-intensity aerobic exercise to improve cognitive performance of children with and without ADHD (Gapin and Etnier, 2010; Hillman et al., 2009; Tantillo et al., 2002), the effect of moderate-intensity, stretching exercise has not been proven yet.
Electroencephalographic studies have reported that most ADHD children display rather consistent differences in brain cortical activity during the resting state compared to normal children. These are typically increased theta in the frontal regions and decreased alpha and beta in the posterior regions (Barry et al., 2003; Chabot and Serfontein, 1996; Loo and Barkley, 2005; Swartwood et al., 2003). A recent study indicated electroencephalographic changes after water aerobic exercise (Huang et al., 2013). Cortical activation patterns have been reported to depend on exercise mode and intensity (Brümmer et al., 2011a; Crabbe et al., 2004; Moraes et al., 2007). Herein, we investigated the effect of acute exercise on the electroencephalography (EEG) of children, with and without ADHD, who participated in a moderate-intensity stretching aerobic exercise program, named health and happiness improving movement (HHIM) exercise (Fig. 1). This exercise regimen was designed to stimulate brain activities, has a metabolic equivalent value of 4.0, and consists of whole-body movements with deep breathing (Ainsworth et al., 2011). We hypothesized that the exercise would alter the cortical activity of children, regardless of their diagnoses. To examine our hypothesis, the EEGs of children were assessed before and after a single exercise bout. In addition, the effects of individual movements on cortical activity were evaluated.
MATERIALS AND METHODSSubjectsTwenty-seven children, 14 boys and 13 girls, that were diagnosed with the combined type of ADHD by their physicians or that met the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for ADHD (American Psychiatric Association, 2000), and 18 healthy control children, 11 boys and seven girls, were recruited from a local public middle school in Seoul, Korea, and participated in the study of individual movements and in the acute exercise study. The ADHD children had no other psychiatric diagnoses or seizure disorder. All children, with and without ADHD, were aged between 12 and 14 yr (12.8±0.79 yr) and their intelligence quotients ranged between 91 and 113 (101.4±10.1) according to the Korean Wechsler Intelligence Scale for Children III. All children were free of pharmacologic treatments for at least 1 month before the testing and during the study period regardless of their previous history of medication. Participation was voluntary and all participants gave written assent and had their parents’ or legal guardians’ written informed consent prior to the experiment. All procedures in the study were approved by the school authority and the HHIM Brain Research Institutional Review Board.
ProcedureTo study the effects of individual movements, the participants in the control and ADHD groups performed two particular movements, D and I (Fig. 1), with an interval of ≥2 hr between each movement to avoid any carryover effects. The participants in the study of acute exercise performed a 13-min exercise bout. A trained physical education teacher supervised the participants.
EEG was measured before and 12±3 min after an acute bout of the exercise. The after-exercise measurements were made during an eyes-closed resting condition in a quiet room while the participants were seated comfortably on a chair. Gold-plated disk electrodes were placed at F7, F8, FZ, CZ, T3, T4, PZ, and OZ, grounded and referenced to A1 and A2, respectively, using a conductive paste (Elefix z-401ce, Nihon Kohden, Irvine, CA, USA) in accordance with the international 10/20 system. Each measurement was carried out after the electroencephalographic signal was stabilized, showing no artifacts for 10 sec. The eyes-closed EEG was also recorded before and immediately after each movement. The participants performed the movements with the electrodes placed on their scalps, and held the postures during the postmovement measurements. The signal was recorded using a QEEG measurement system (PolyG-I, Laxtha Inc., Daejeon, Korea) with a sampling rate of 256 Hz and a frequency filter set at 0.5–50 Hz, converted and stored for off-line analysis using TeleScan ver. 3.03 (Laxtha Inc.). All electroencephalographic data were visually inspected to reject artifacts, and were subjected to a fast Fourier transform power spectral analysis to obtain absolute power estimates in the theta (3.5–8 Hz), alpha (8–12 Hz), sensory motor rhythm (SMR) (12–16 Hz), and beta (16–25 Hz) bands. Relative power, i.e., the absolute power in a band expressed as a percentage of the total power across the region of 3.5–25 Hz, was calculated for each band and subjected to statistical analysis. For simplicity, the power distributions before acute exercise are labeled as before-exercise distributions in Fig. 2.
Data analysisAll statistical analyses were conducted with a significance level (alpha) of 0.05 using IBM SPSS Statistics ver. 19.0 (IBM Co., Armonk, NY, USA). The effects of individual movements were analyzed for each frequency band using two-way analyses of variance (ANOVAs) with movement (before/after) and group (ADHD/control children, ADHD boys/ADHD girls, or control boys/control girls) as factors and the power amplitude (absolute or relative) at each electrode site as variable. Analyses of the acute exercise effects were conducted using repeated-measures ANOVAs with exercise (before/after) as within-subjects factor, group (ADHD/control children, ADHD boys/ADHD girls, or control boys/control girls) as between-subjects factor, and the power amplitude (absolute or relative) at each electrode site as within-subjects variable. Data presented herein are with a significance level (P) of <0.05 unless otherwise indicated.
RESULTSEffect of acute exercise
Fig. 2 shows the topographic distributions of the relative electroencephalographic power of children in the control and ADHD group before and after a single bout of exercise. The relative electroencephalographic activities in the leftmost columns of Fig. 2 display that the ADHD children exhibited globally higher relative theta before exercise, especially in the frontal and central regions, and lower relative alpha along the midline regions as compared with the healthy controls. Relative SMR and beta activities of the ADHD children were also lower compared with the healthy children. After exercise, both control and ADHD children exhibited decreased relative theta, and increased alpha, SMR, and beta activities over the entire regions as shown in the center columns of Fig. 2. The electroencephalographic power of the children in control and ADHD group before and after a single bout of exercise, exhibiting statistically significant main effects of the exercise. The exercise produced electroencephalographic changes in the theta and alpha bands of both absolute and relative power, but mostly of relative power. In both groups, the exercise reduced absolute theta in the left frontal region and relative theta along the midline regions, and elevated absolute alpha in the central and posterior regions and relative alpha globally. It was notable that the increases in relative alpha were substantial not only in the ADHD group but also in the controls. No significant interaction effects of exercise and group appeared. Within the ADHD group, the girls had less overall relative alpha than the boys at baseline. The exercise had main effects on both boys and girls, elevating relative alpha, with no significant interaction of exercise and gender. On the other hand, significant interaction effects of exercise and group appeared in the control group. As compared with their counterparts, the girls also had less baseline alpha that increased more substantially after exercise.
Effect of individual movements
Fig. 3 presents relative electroencephalographic power in the theta and beta bands of the children in the control and ADHD group before and immediately after movement. After movement D, both control and ADHD children exhibited increased theta. The main effect of movement emerged in the midline frontal region (F [1, 154]=4.046, P=0.046). Although the data are not shown in Fig. 3, the main effect of movement also emerged in the midline central (F [1, 154]=9.601, P=0.002), parietal (F [1, 154]=13.55, P<0.0001), and occipital (F [1, 154]=12.23, P=0.001) regions. The main effect of group appeared in the midline frontal (F [1, 154]=6.052, P=0.015), and also in the parietal (F [1, 154]=4.602, P=0.034) and occipital (F [1, 154]=5.679, P=0.018) regions (not shown in Fig. 2). No interaction effects of movement and group appeared. On the other hand, after movement I, both control and ADHD children exhibited decreased relative theta. The main effect of movement appeared in the left (F [1, 154]=4.956, P=0.027) and midline frontal (F [1, 154]=7.421, P=0.007) regions (Fig. 3). No significant effect of group or interaction of movement and group appeared. Within the ADHD group, the girls had more relative theta than the boys at baseline. Movement I reduced the theta activity of both boys and girls, but more drastically of the girls, exhibiting an interaction of movement and gender in the midline frontal region. Within the control group, no statistically significant effect of movement or group emerged.
Relative beta increased in both control and ADHD children, exhibiting the main effect of movement in the midline frontal (F [1, 154]=5.005, P=0.027), central (F [1, 154]=8.356, P=0.004), parietal (F [1, 154]=13.434, P<0.0001), and posterior (F [1, 154]= 17.393, P<0.0001) regions for movement D, and left (F [1, 154]= 5.537, P=0.02), right (F [1, 154]=5.131, P=0.025), midline frontal (F [1, 154]=22.06, P<0.0001), central (F [1, 154]=27.30, P< 0.0001), parietal (F [1, 154]=33.30, P<0.0001), and posterior (F [1, 154]=19.69, P<0.00010) regions for movement I (Fig. 3). The main effect of group appeared in the left (F [1, 154]=6.387, P= 0.013), right (F [1, 154]=8.784, P=0.004), and midline frontal (F [1, 154]=5.947, P=0.016) for movement D, and midline frontal (F [1, 154]=5.107, P=0.025), and parietal (F [1, 154]=5.497, P=0.02) regions for movement I (Fig. 3). Although the data are not shown in Fig. 3, for both movements the main effect of group appeared in the left temporal region (D: F [1, 154]=6.036, P=0.015; I: F [1, 154]=5.040, P=0.026). No interaction of movement and group emerged for both movements. Within each group, the ADHD and control group, the main effect of movement I appeared for the boys and girls, with no interaction effect of movement and gender. Movements D and I reduced relative alpha globally in the control and ADHD children, exhibiting main effects of movement with no interaction of movement and group, as shown in Fig. 4. The main effect of group appeared for movement D in the parietal region. Within the ADHD group, both movements reduced relative alpha of the boys and girls with no significant interaction effects of movement and gender (Fig. 5). Similarly, the movements affected the alpha activity of the boys and girls in the control group.
Fig. 6 presents the theta-to-beta ratios of the children before and immediately after movement I. Compared with the healthy controls, the ADHD children had higher theta-to-beta ratios in the frontal, midline central and parietal regions at baseline. The main effect of movement appeared in these regions with no interaction of movement and group, reducing the ratios in both control and ADHD children. The main effect of group appeared in the parietal region. Within the ADHD group, the girls had higher theta-to-beta ratios than the boys at baseline, particularly in the frontal regions (Fig. 7). It appears that movement I reduced the ratios in the girls more substantially as compared with the boys. However, neither statistically significant gender effect nor interaction of movement and gender appeared.
DISCUSSIONIn this study, we evaluated the effects of HHIM exercise, an aerobic stretching exercise program, on the EEG of children. Before exercise, compared with the healthy controls, the ADHD children had globally elevated relative theta, reduced relative alpha along the midline regions in particular, and reduced relative SMR and beta, which is consistent with the previous findings (Barry et al., 2003; Loo and Barkley, 2005; Swartwood et al., 2003). After a single bout of the exercise, all children exhibited electroencephalographic changes, regardless of their diagnoses, mostly with theta decreasing and alpha increasing. Electroencephalographic studies have revealed that, during the course of the central nervous system (CNS) maturation of children, alpha activity increases and theta decreases with age, and that changes in cortical arousal are reflected in the theta and beta bands (Benninger et al., 1984; Clarke et al., 2001; Lubar, 1991). Based on this, the effects of stimulant medications, producing changes primarily in the theta and beta bands, have been attributed to the promotion of cortical arousal rather than the changes in the maturation status of the ADHD brain (Clarke et al., 2002). In contrast, the HHIM exercise affected theta and alpha, indicating its beneficial effects on brain maturation and development of children, especially those with ADHD. Recent brain imaging studies have confirmed the cortical maturation delay in children with ADHD (Shaw et al., 2007; Shaw et al., 2012).
It has been reported that the girls (ages 8 to 12 yr) have more absolute and relative theta and less relative alpha than the age-matched boys, indicating a developmental lag in their EEG (Benninger et al., 1984; Clarke et al., 2001). In this study, we also found such gender differences in children. At baseline, the girls had much less relative alpha than their counterparts, regardless of their diagnoses. The acute exercise affected the healthy girls in the control group, elevating their alpha more drastically compared with the boys. This sheds light on the rather substantial increase in relative alpha of the control children, showing no differential effects of exercise between the control and ADHD children. The exercise significantly elevated alpha in both boys and girls with ADHD, with no gender-dependent differential effects. These findings support that healthy girls (as compared to healthy boys) and ADHD children all have a developmental lag in the EEG and that the HHIM exercise facilitates CNS maturation. Further studies are needed to investigate the effects of chronic HHIM exercise.
Previous studies reported that dominant electroencephalographic activity is in the theta and alpha regions when one is at rest, which shifts toward the beta region when excited and that the alpha activity is inversely related to cortical activation (Feige et al., 2005; Lubar, 1991). In line with this, we found that the individual movements, D and I, reduced relative alpha and elevated relative beta activity of the children in both the control and ADHD groups. The impact of these movements on relative alpha was about the same in the entire region but was different on relative beta, i.e., movement D affected relative beta along the midline and movement I in the entire region. It was intriguing to observe that relative theta was elevated by movement D but reduced by movement I. Accordingly all children exhibited reduced theta-to-beta ratios after movement I. In the ADHD group, the effects of movement I were found to be independent of gender on the alpha and beta bands and on the theta-to-beta ratios but were gender-dependent on relative theta. Studies on individual movements have been limited to hand or finger movements due to difficulties involved in assessing complex whole-body movements (Brümmer et al., 2011b). It is, therefore, noteworthy that in the present study differential effects of individual whole-body movements were observed on the cortical activity of children. This warrants an investigation of the individual effects of the entire HHIM exercise movements to better understand their collective effects. It is noted that most studies to date have been carried out to build exercise regimens for healthy adults but not for children or adolescents, especially those with mental disorders. To this end, the present study may provide a basis for tailoring exercise to individual needs, to particularly develop a viable, non-pharmacologic treatment strategy for ADHD.
In summary, the present study uncovers the positive effects of an aerobic stretching exercise on the cortical activity of children with and without ADHD. The exercise exhibited acute effects, particularly affecting ADHD children toward normalization of relative theta and alpha. The examination of individual movements indicated their differential effects on cortical activity of children. Although speculative, we may attribute the positive effects observed herein to the individual whole-body movements of the HHIM exercise in a concerted way stimulating the brain activity, more so than other aerobic exercise modalities such as walking, running or cycling.
ACKNOWLEDGMENTSThis study was sponsored by a grant from the HHIM Brain Research Institute. We thank all children and their families for participation.
REFERENCESAinsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Text Revision. Washington, DC: American Psychiatric Association; 2000.
Barbaresi WJ, Colligan RC, Weaver AL, Voigt RG, Killian JM, Katusic SK. Mortality, ADHD, and psychosocial adversity in adults with childhood ADHD: a prospective study. Pediatrics. 2013;131:637–644.
Barry RJ, Clarke AR, Johnstone SJ. A review of electrophysiology in attention-deficit/hyperactivity disorder: I. Qualitative and quantitative electroencephalography. Clin Neurophysiol. 2003;114:171–183.
Benninger C, Matthis P, Scheffner D. EEG development of healthy boys and girls. Results of a longitudinal study. Electroencephalogr Clin Neurophysiol. 1984;57:1–12.
Brümmer V, Schneider S, Abel T, Vogt T, Strüder HK. Brain cortical activity is influenced by exercise mode and intensity. Med Sci Sports Exerc. 2011a;43:1863–1872.
Brümmer V, Schneider S, Strüder HK, Askew CD. Primary motor cortex activity is elevated with incremental exercise intensity. Neuroscience. 2011b;181:150–162.
Bush G. Cingulate, frontal, and parietal cortical dysfunction in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2011;69:1160–1167.
Chabot RJ, Serfontein G. Quantitative electroencephalographic profiles of children with attention deficit disorder. Biol Psychiatry. 1996;40:951–963.
Clarke AR, Barry RJ, Bond D, McCarthy R, Selikowitz M. Effects of stimulant medications on the EEG of children with attention-deficit/hyperactivity disorder. Psychopharmacology (Berl). 2002;164:277–284.
Clarke AR, Barry RJ, McCarthy R, Selikowitz M. Age and sex effects in the EEG: development of the normal child. Clin Neurophysiol. 2001;112:806–814.
Crabbe JB, Dishman RK. Brain electrocortical activity during and after exercise: a quantitative synthesis. Psychophysiology. 2004;41:563–574.
Cubillo A, Halari R, Giampietro V, Taylor E, Rubia K. Fronto-striatal underactivation during interference inhibition and attention allocation in grown up children with attention deficit/hyperactivity disorder and persistent symptoms. Psychiatry Res. 2011;193:17–27.
Feige B, Scheffler K, Esposito F, Di Salle F, Hennig J, Seifritz E. Cortical and subcortical correlates of electroencephalographic alpha rhythm modulation. J Neurophysiol. 2005;93:2864–2872.
Foley TE, Fleshner M. Neuroplasticity of dopamine circuits after exercise: implications for central fatigue. Neuromolecular Med. 2008;10:67–80.
Gapin J, Etnier JL. The relationship between physical activity and executive function performance in children with attention-deficit hyperactivity disorder. J Sport Exerc Psychol. 2010;32:753–763.
Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: can we alter the developmental trajectory of ADHD? Neurosci Biobehav Rev. 2011;35:621–634.
Hazell P. The challenges to demonstrating long-term effects of psychostimulant treatment for attention-deficit/hyperactivity disorder. Curr Opin Psychiatry. 2011;24:286–290.
Hillman CH, Pontifex MB, Raine LB, Castelli DM, Hall EE, Kramer AF. The effect of acute treadmill walking on cognitive control and academic achievement in preadolescent children. Neuroscience. 2009;159:1044–1054.
Huang CJ, Hung CL, Huang CW, Hung TM. Effects of eight weeks physical activity intervention on resting EEG in children with attention deficit hyperactivity disorder. Psychophysiology. 2013;50:S30
Loo SK, Barkley RA. Clinical utility of EEG in attention deficit hyperactivity disorder. Appl Neuropsychol. 2005;12:64–76.
Loo SK, Teale PD, Reite ML. EEG correlates of methylphenidate response among children with ADHD: a preliminary report. Biol Psychiatry. 1999;45:1657–1660.
Lubar JF. Discourse on the development of EEG diagnostics and biofeedback for attention-deficit/hyperactivity disorders. Biofeedback Self Regul. 1991;16:201–225.
Moraes H, Ferreira C, Deslandes A, Cagy M, Pompeu F, Ribeiro P, Piedade R. Beta and alpha electroencephalographic activity changes after acute exercise. Arq Neuropsiquiatr. 2007;65:3A. 637–641.
Seidman LJ, Valera EM, Makris N. Structural brain imaging of attention-deficit/hyperactivity disorder. Biol Psychiatry. 2005;57:1263–1272.
Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, Clasen L, Evans A, Giedd J, Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proc Natl Acad Sci U S A. 2007;104:19649–19654.
Shaw P, Malek M, Watson B, Sharp W, Evans A, Greenstein D. Development of cortical surface area and gyrification in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2012;72:191–197.
Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046.
Swartwood JN, Swartwood MO, Lubar JF, Timmermann DL. EEG differences in ADHD-combined type during baseline and cognitive tasks. Pediatr Neurol. 2003;28:199–204.
Tantillo M, Kesick CM, Hynd GW, Dishman RK. The effects of exercise on children with attention-deficit hyperactivity disorder. Med Sci Sports Exerc. 2002;34:203–212.
U.S. Food and Drug Administration. FDA asks attention-deficit hyperactivity disorder (ADHD) drug manufacturers to develop patient medication guides [Internet]. Silver Spring (MD): U.S. Food and Drug Administration; 2013. [updated 2013 Aug 22; cited 2016 Feb 12]. Available from: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm107918.htm.
Fig. 1Exemplary movements and postures included in health happiness improvement movement exercise. Insets show side views. 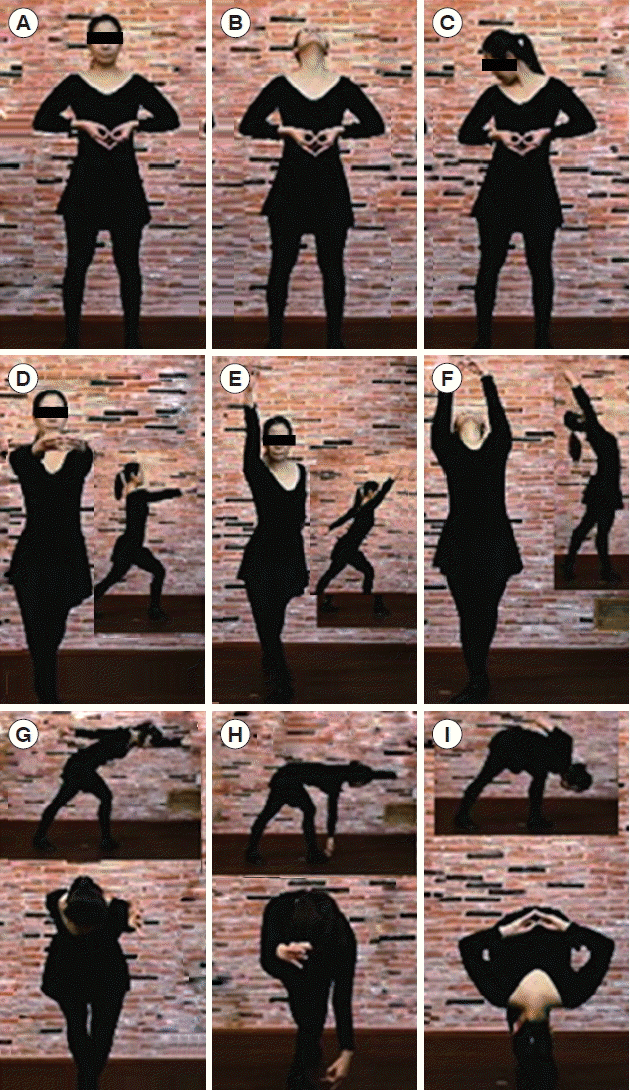
Fig. 2Topographic distributions of relative electroencephalography power for children in control (A) and attention-deficit/hyperactivity disorder (ADHD) (B) group. The left and right columns show the maps of children before acute exercise and after acute exercise, respectively. SMR, sensory motor rhythm. 
Fig. 3Relative theta and beta power of children before and immediately after movement, displaying main effects of movement (M) and group (G) (P<0.05). D, movement D; I, movement I; ADHD, attention-deficit/hyperactivity disorder. *P<0.0001. 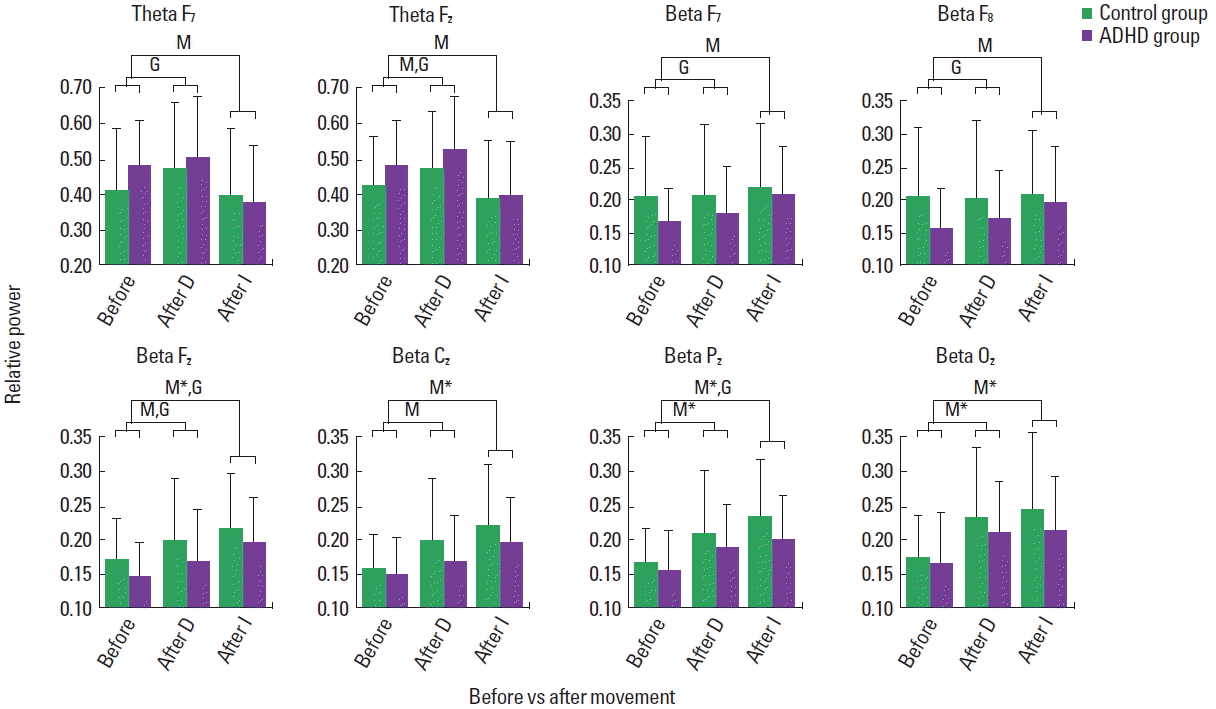
Fig. 4Relative alpha power of children in attention-deficit hyperactivity disorder (ADHD) and control group, before and immediately after movement, displaying main effects of movement (P<0.0001). No interaction of movement and group appeared. D, movement D; I, movement I. *Mean effects of group also appeared (P=0.029). 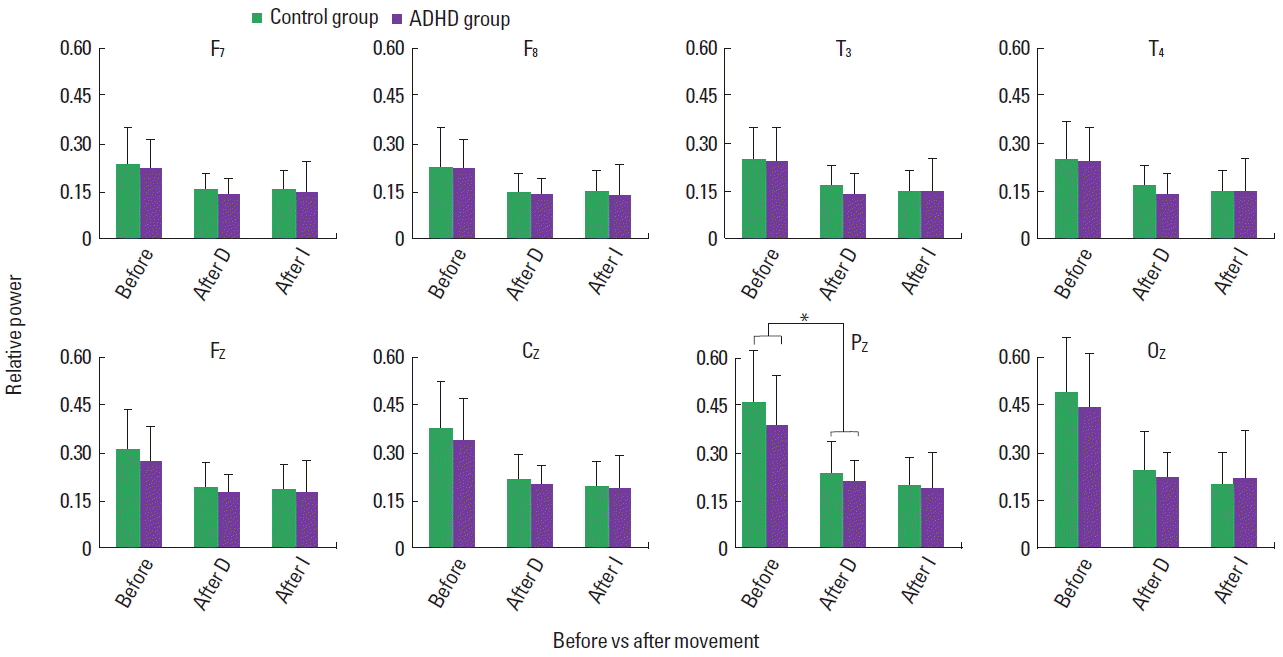
Fig. 5Relative alpha power of attention-deficit hyperactivity disorder (ADHD) boys and girls before and immediately after movement, displaying main effects of movement (P<0.0001). No gender effect or its interaction with movement appeared. D, movement D; I, movement I. *P=0.001. 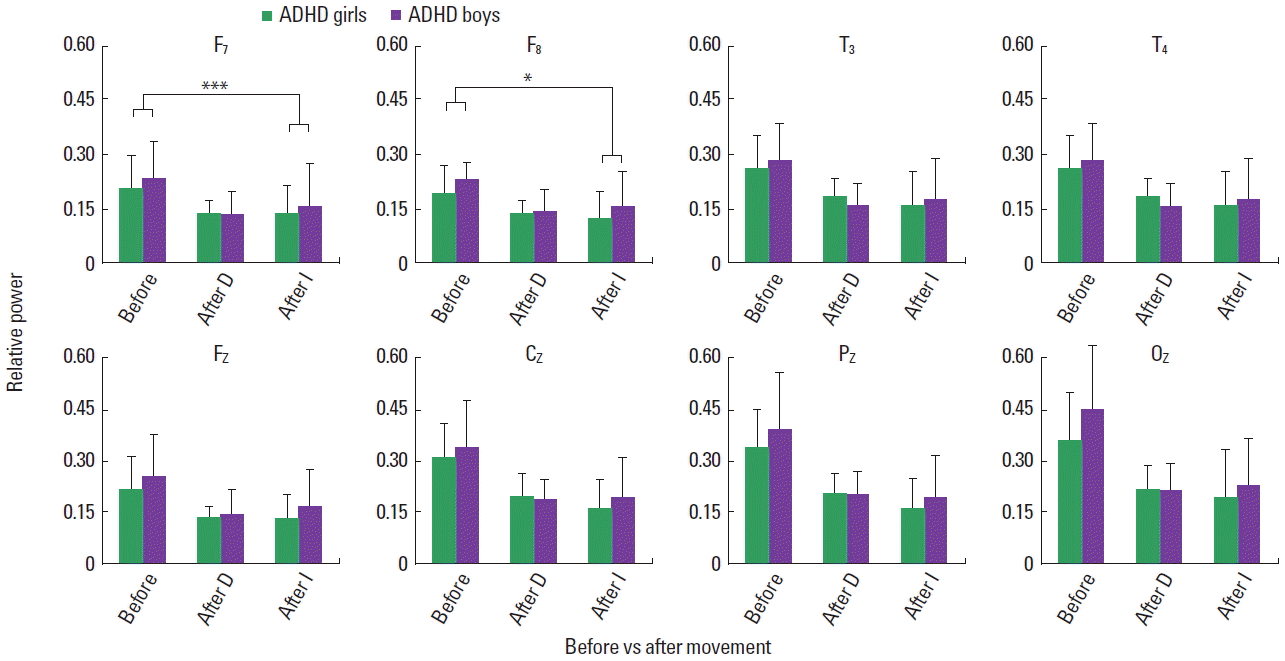
|
|
|||||||||||||||||||||||||||||||||||||||||||