AbstractThe purpose of this study was to investigate the expression of lipogenesis- and lipolysis-related genes and proteins in skeletal muscles after 12 weeks of resistance training. Sprague-Dawley rats (n=12) were randomly divided into control (resting) and resistance training groups. A tower-climbing exercise, in which rats climbed to the top of their cage with a weight applied to their tails, used for resistance training. After 12 weeks, rats from the resistance training group had lower body weights (411.66±14.71 g vs. 478.33±24.63 g in the control), there was no significant difference between the two groups in the concentrations of total cholesterol, and high or low density lipoprotein cholesterol. However, the concentration of triglyceride was lower in resistance-trained rats (59.83±14.05 μg/mL vs 93.33±33.89 μg/mL in the control). The mRNA expression levels of the lipogenesis-related genes sterol regulatory element binding protein-1c, acetyl-CoA carboxylase, and fatty acid synthase were not significantly different between the resistance-trained and control rats; however, mRNA expression of the lipolysis-related carnitine palmitoyl transferase 1 and malonyl-CoA decarboxylase increased significantly with resistance training. AMP-activated protein kinase protein levels also significantly increased in resistance training group compared with in the control group. These results suggested that resistance exercise training contributing to reduced weight gain may be in part be due to increase the lipolysis metabolism and energy expenditure in response to resistance training.
INTRODUCTIONTaking exercise, particularly aerobic exercise, is a low-cost therapeutic lifestyle change that has been recommended for improving lipid and lipoprotein levels in adults (Rosamond et al., 2008). The improvements in lipoprotein-lipid profiles with aerobic training (Booth and Baldwin, 1996; Coon et al., 1989; Lokey and Tran, 1989; Poirier et al., 1996) are reportedly due primarily to an accompanying loss of fat mass that results from increased fat oxidation with exercise (Jeukendrup, 2002). Aerobic exercise has also been shown to increase the following: AMP-activated protein kinase (AMPK) with the increasing translocation of glucose transporter type 4 to the plasma membrane and glucose uptake (Hayashi et al., 2000), carnitine palmitoyl transferase 1 (CPT-1) as the rate-limiting enzyme for fatty acid oxidation (McGarry and Brown, 1997; Melanson et al., 2010), and the first step specific to the fatty acid oxidation process (Braith and Stewart, 2006); sterol regulatory element binding protein-1c (SREBP-1c) as a transcription factor that regulates lipogenic gene expression related to fatty acid synthase (FAS), malonyl-CoA, and acetyl-CoA carboxylase (ACC) (Brown and Goldstein, 1997; Ikeda et al., 2002). In addition, various transcriptional coactivators and transcription factors are known to control fatty acid β-oxidation and intramuscular triglyceride (TG) transport (Russell et al., 2005).
In contrast to the effects of aerobic exercise, the effects of resistance training (i.e., strength or weight training) on the lipoprotein-lipid profile did not well documented. Resistance training is suggested to be effective for increasing muscle mass (Trevisan et al., 2010; Warburton et al., 2001), muscle strength (Lima et al., 2009), bone mineral density (American College of Sports Medicine et al., 2009; Lima et al., 2009), and for reducing total and relative body fat stores (Donnelly et al., 2003; Rabelo et al., 2011) 12. However, the reported effects of resistance training on lipids and lipoprotein are inconsistent. For example, some previous studies have shown that resistance training reduces total cholesterol (TC), TG, and low-density lipoprotein cholesterol (LDL-C), and increases high-density lipoprotein cholesterol (HDL-C) (Castaneda et al., 2002; Fahlman et al., 2002; Kelley and Kelley, 2009). Whereas, other studies have reported that lipids and lipoproteins remain unchanged after resistance training (Banz et al., 2003; Elliott et al. 2002; Vincent et al., 2003 ).
Skeletal muscle, which accounts for 30%–40% of body mass in mammals, is an important site for glucose clearance, lipid oxidation, and thermogenesis. It is a flexible tissue capable of altering the type and level of proteins expressed in response to complex processes and specific signaling mechanisms (Bouchard et al., 1997). Exercise-induced adaptation in skeletal muscle results in a variety of metabolic changes including cellular biogenesis (Irrcher et al., 2003) and subsequent protein synthesis (Booth and Baldwin, 1996). Endurance exercise training increases the capacity for fat oxidation by increasing mitochondrial density in skeletal muscle as well as the gene expression and protein content of free fat acid (FFA) transporters, which may assist in the uptake and delivery of FFA to the mitochondria. However, the effects of lipid metabolism in skeletal muscle after resistance training are currently unclear. For example, the physiological role played by malonyl-CoA decarboxylase (MCD) in the regulation of malonyl-CoA (which is involved in fatty acid biosynthesis) remains open to question, as are the mechanisms by which its activity regulated.
Based on the aforementioned knowledge gaps, the aims of this study were as follows. First, the metabolic effects of resistance training on the transcriptional regulation of lipid metabolism-related enzymes (i.e., those involved in lipogenesis and lipolysis) investigated in the skeletal muscle of rats. Second, changes in body composition and plasma lipid concentrations (i.e., for TC, TG, LDL-C, and HDL-C) due to lipid metabolism during resistance training were also assessed in the same animals.
MATERIALS AND METHODSExperimental animals and treatmentTwelve male Sprague-Dawley rats (203±12.43 g; DBL, Eumseong, Korea) housed in plastic cages at 7 weeks of age. The animals were housed in colony cages in a controlled environment with a maintained temperature (24°C) and humidity (56%), and a 12:12-hr light:dark cycle (dark cycle was from 12:00 a.m. until 12:00 p.m.). All rats had free access to water and food. The 12 rats were randomly divided into two groups designated as the rest (CON, n=6) and resistance training (RT, n=6) groups. The experimental protocol developed according to the guidelines of the Korean Science Academy for the care and use of laboratory animals.
Resistance training programFor resistance training, a tower climbing exercise used. This involved climbing a 1.35-m-high ladder with 2.5-cm grid steps and a 60° gradient. This equipment previously applied by Lee et al. (2004). In the first week of training, rats familiarized with the process of climbing to the top of their cage with a weight load (plastic bolt) attached to their tail that represented 50% of their body weight. Training took place on three alternating days (one day with training, one day without). After this first week of adaptation, the one-rep maximum (1RM) of exercised rats was calculated. Training sessions from the second weeks and for the next 8 weeks, involved training with intensities at 50%, 75%, 90%, and 100% of each rat’s 1RM. If, during these 8 weeks, a rat was able to complete eight climbs each with an increasing weight load, the training session considered to have been performed from the bottom to the top, and 30 g of extra weight was added to the next trial period from 9 to 12 weeks. Once a rat had climbed from the bottom to the top of the ladder, they had given 2 min of rest before the next trial.
Sample preparationRats were sacrificed 24 hr after their last bout of exercise. On the day of sacrifice, rats anaesthetized with chloroform and blood collected from the left ventricle. Tissue from skeletal muscle, specifically the biceps, collected and weighed. These samples were immediately frozen in liquid nitrogen and stored at −80°C to await analysis.
Lipid profilesBlood collected and centrifuged at 3,000 rpm for 10 min at 4°C, and the resultant serum was stored at −20°C until use. TG, TC, and HDL-C analyzed using the enzymatic colorimetric method on a COBAS integra 800 instrument (Roche, Basel, Switzerland). LDL-C calculated as follows using the Friedewald equation (1972): LDL-C=TC–HDL-C–TG/5.
RNA isolation and reverse transcription polymerase chain reactionTotal RNA from the biceps was isolated using RibospinTM (GeneAll, Seoul, Korea). Total RNA quantified at 260-nm absorption by using a NanoVue Spectrophotometer (GE Healthcare, Freiburg, Germany). mRNA expression levels were measured using a reverse transcription polymerase chain reaction (RT-PCR) machine (Labnet, Woodbridge, NJ, USA), and RT-PCR was conducted using 1 μg of denaturated RNA and PCR premix (Bioneer, Seoul, Korea). Forward and reverse primers obtained from Bioneer, and their sequences were as follows: β-actin forward primer, 5′-CGTAAAGACCTCATAGCCAA-3′; β-actin reverse primer, 5′-AGCCATGCCAAATGTGTCAT -3′; ACC-2 forward primer, 5′-ACAGTGAAGGCTTACGTCTG-3′; ACC-2 reverse primer, 5′-AGG ATCCTTACAAC-CTCTGC-3′; FAS forward primer, 5′-TGTCAACCGTGTCAGCCTG-3′; FAS reverse primer, 5′-TGGATGATGTTGATGATAGAC-3′; SREBP-1c forward primer, 5′-GGA GCCATGGATTGCACATT-3′; SREBP-1c reverse primer, 5′-AGGAAGGCTTCCAGAGAGGA-3′; CPT-1 forward primer, 5′-GGAGACAGACACCATCCAACATA-3′; CPT-1 reverse primer, 5′-AGGTGATGGACCTTGTCAACCC-3′; lipoprotein lipase (LPL) forward primer, 5′-ACCAT CAGGATATAGCACCC-3′; LPL reverse primer, 5′-ACCATCAGGATATAGCACCC-3′; MCD forward primer, 5′-CCGCTGGAACATCACTTTG-3′; MCD reverse primer, 5′-GGGGATACGG CTGGAT-3′. The signal from the housekeeping gene β-actin used for normalization. The band density of PCR confirmed by electrophoresis on a 6% agarose gel.
Western blot analysisSkeletal muscle tissue was homogenized in Triton lysis buffer (20-mM Tris, pH 7.4; 137-mM NaCl; 25-mM β-glycerophosphate, pH 7.14; 2-mM sodium pyrophosphate; 2-mM ethylene-diaminetetraacetic acid; 1-mM Na3VO4; 1% Triton X-100; 10% glycerol; 5-μg/mL leupeptin; 5-μg/mL aprotinin; 3-μM benzamidine; 0.5-mM dithiothreitol; and 1-mM phenylmethanesulfonyl fluoride. The protein concentration of the supernatants measured using the Bradford method (1976). Proteins (50 μg in each lane), ACC and AMPK, were separated using 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred to a polyvinylidene difluoride membrane (Pall Corp., Port Washington, NY, USA) from Millipore. Membrane blocking was performed by using a blocking buffer (34-mL Tris-buffered saline and 1.05-g bovine serum albumin) at room temperature for 1 hr. Rabbit polyclonal antibody (p-ACC Ser; Cell Signaling Technology, Beverly, MA, USA) and anti-AMPK antibody (400064; Calbiochem, SanDiego, CA, USA) were used following dilution in blocking solutions at 1:1,000 dilutions. After washing, an horseradish peroxidase-conjugated secondary antibody added and the blot incubated for 30 min at room temperature. The membrane then washed, and the bands were visualized using electrochemiluminescence (Amersham Biosciences UK Ltd, Giles, UK). The films were scanned with a Sharp JX-330 scanner, and protein bands were quantified using Image Master v3.0 analysis software (Amersham Pharmacia Biotech, Piscataway, NJ, USA).
Statistical analysisStatistical analyses conducted using SPSS ver. 17.0 (SPSS Inc., Chicago, IL, USA). In this report, all values expressed as mean±standard deviation. The independent t-test were used to analyze weight differences between the CON and RT groups during the 12-week training period, and to determine significant differences in lipogenesis and lipolysis variables between groups. P<0.05 considered statistically significant.
RESULTSChanges in body weight of rats during 12 weeks resistance training
Table 1 shows the changes in body weight observed in rats during the 12 weeks of resistance training. Analysis showed that there was an interaction between the CON and RT groups (F [5, 50]=28.795, P<0.001). At the baseline (0 week), the body weight of rats in the CON (194.16±13.93 g) and RT (198.33± 5.16 g) groups were similar. There was a significant difference in body weight observed between the two groups at 4 weeks, with body weight significantly lower in the RT group (P<0.001). This difference maintained until the end of training at 12 weeks.
Blood lipid profiles in rats after 12-week resistance training
Table 2 shows blood lipid profiles in rats after 12-week resistance training. No significant differences in TC, HDL-C, or LDL-C observed between the CON and RT groups. However, rats in the RT group showed significantly lower TG levels than those in the CON group (P<0.05).
Lipogenesis- and lipolysis-related mRNA and protein expression in rat muscle tissueThe level of lipogenesis gene mRNA observed in muscle tissue was not significantly different between the RT and CON group (Fig. 1). However, for lipolysis-related genes, CPT-1 (P<0.05) and MCD (P<0.001), mRNA levels significantly increased in the RT group compared with those observed in the CON group (Fig. 2). In addition, AMPK protein levels (also related to lipolysis) were significantly higher in the RT group compared with AMPK levels in the CON group (P<0.001) (Fig. 3).
DISCUSSIONResistance training is associated with a significant decrease in body weight and percentage body fat resulting from increased energy expenditure due to increased fat free mass. In previous studies, resistance training also increased fat oxidation during rest and exercise, which might account for the associated decrease in body weight and percentage body fat (Washburn et al., 2012). In the present study, body weight was significantly lower in RT group compared with in the CON group. This result observed after 4 weeks of training and maintained throughout the training period. Therefore, resistance training controlled the rats’ weight may be due to increased lipolysis metabolism and energy expenditure in response to resistance training (Miyazaki et al., 2001).
During resistance exercise, a large amount of energy utilized by muscle contractions. Glycogen is a primary intramuscular energy source in skeletal muscle; however, these muscles also contain TG, which is another energy-rich substrate that may be used during prolonged exercise (Ikeda et al., 2002). Schrauwen et al. (1998) reported that the reduction in muscle glycogen storage after exercise was the major mediator of enhanced fatty acid oxidation. An exercise-mediated increased in AMPK activity can also increase fat oxidation (Merrill et al., 1997) as well as block TG synthesis in skeletal muscle (Muoio et al., 1999). Yang et al. (2006) found that 8 weeks of resistance training in rats significantly reduced weight gain and increased TG concentration in serum but had no effect on TC, HDL-C, and LDL-C. They suggested that changes in TG concentration facilitated an increase in fatty acid via TG for the purposes of supercompensation, which functioned to provide energy to the skeletal muscle. Similarly, we found that TC, HDL-C, and LDL-C concentrations were not affected by resistance training; however, in contrast to Yang et al. (2006), we observed significantly lower TG concentrations in rats to resistance training compared with those in the control group. Nadeau et al. (2006) suggested that lipogenesis-related gene expression may be enhanced by the compensatory interaction of lipid metabolism during the early exercise period, and that this gene expression later decreases to adjust for prolonged periods of resistance training. Therefore, we suggest that lipogenesis-related gene expression was not affected by resistance training in our study because lipid metabolism may have adapted during the relatively long 12-week period of resistance exercise training.
A family of membrane-bound transcription factors, the SREBPs, regulates lipid homeostasis in vertebrate cells. SREBPs directly activate the expression of more than 30 genes dedicated to the synthesis and uptake of cholesterol, fatty acids, TGs, and phospholipids, as well as the nicotinamide adenine dinucleotide phosphate cofactor required to synthesize these molecules (Edwards et al., 2000; Sakakura ret al., 2001). Of the three SREBP isoforms, SREBP-1c preferentially enhances transcription of genes required for fatty acid synthesis, but not cholesterol synthesis, in the liver, adipose tissue, and skeletal muscle (Brown and Goldstein 1997; Guillet-Deniau et al., 2002; Muoio et al., 1999). SREBP-1c is a key transcription factor for up-regulation of lipogenic pathway genes, such as ACC and FAS, and down-regulation of lipolysis pathway genes, such as CPT-1, in the skeletal muscle. Previous studies have demonstrated that SREBP-1c and FAS activation increases in skeletal muscle after endurance exercise (Goodpaster et al., 2001; Nadeau et al., 2006) and swimming training (Ikeda et al., 2002). However, in the present study, mRNA expression of SREBP-1c, FAS, and ACC was not significantly different between rats subjected to resistance training and those in the rest-control group. These differences may be attributable to differences in exercise type, duration, and intensity between the various training programs.
Although endurance training affects lipogenesis gene expression, the effects of resistance training, e.g., climbing exercises, on the expression of these genes appear to be inconsistent (Kelley and Kelley, 2009; Lokey and Tran, 1989). In addition, endurance training results in higher oxidative stress than resistance training, and it increases lipolysis gene expression because the exercise stimulates TG replenishment in skeletal muscle, which in turn up-regulates SREBP-1c leading to an increase in ACC and FAS mRNAs. This could explain why these changes appear in the biological response to endurance training rather than that of resistance training. Another reason could be the control of diet, i.e., experimentation with or without dietary restrictions. Here, we did not restrict the calorie intake of rats during the training period; however, Nadeau et al. (2006) reported that IMTG increased when calories were restricted, which may be an important biological defense mechanism in response to limited glycogen stores. In this reason, this experiment may not need to be increasing TG for fuel, so it has less effects lipogenesis pathway.
Malonyl-CoA is regulated by the enzyme MCD, which converts malonyl-CoA back to acetyl-CoA. Genetic knockdown of MCD results in elevated malonyl-CoA levels and reduced rates of fatty acid oxidation in both human and rodent skeletal muscle (Bouzakri et al., 2008; Koves et al., 2008). MCD activity is enhanced by AICAR and contractions in skeletal muscle (Kuhl et al., 2006; Park et al., 2002), and increased MCD activity can be attributed to the activation of AMPK, an enzyme that phosphorylates and inhibits ACC (Saha et al., 2000). CPT-1, an enzyme that controls the transfer of long-chain fatty acyl CoA into mitochondria, also inhibits ACC and FAS, and it decreased in malonyl-CoA and increased in CPT-1 (Ruderman et al., 2003). In our study, the activity of the lipolysis-related enzymes CPT-1 and MCD increased after resistance training; thus, we suggest that resistance training may stimulate CPT-1 by enhanced β-oxidation.
Our results also showed that AMPK activity significantly increased following resistance training. AMPK has been proposed as a key molecule for eliciting metabolic adaptations to exercise and it is an important regulator of the energy consumption and regeneration pathway (Sakamoto and Goodyear, 2002). Moreover, AMPK is a multisubunit enzyme that recognized as a major regulator of the lipid biosynthetic pathway because of its role in the phosphorylation and inactivation of key enzymes such as ACC (Hardie and Carling, 1997). AMPK mediates a decrease in SREBP-1 mRNA and protein expression, and AMPK phosphorylation inhibits ACC and FAS, which is the rate-limiting enzyme for malonyl-CoA synthesis (Foretz et al., 1998; Woods et al., 2000), and increases CPT-1, the activation of which increases fatty acid oxidation (Assifi et al., 2005).
In this study, despite observing increased AMPK activity in the skeletal muscles of rats after resistance training, the expression of lipogenesis genes SREBP-1c, ACC2, and FAS not affected by resistance training. However, the expression of the lipolysis genes CPT-1 and MCD increased after resistance training along with AMPK activity. The resistance exercise apparently increased fatty acid oxidation; Here, in addition to increased lipolysis gene expression, AMPK protein levels increased in rats after resistance training. Therefore, we suggest that resistance training affected lipolysis-related protein and enzyme activity in skeletal muscle, and that this lead to increased lipid metabolism and reduced weight gain in rats.
REFERENCESAmerican College of Sports Medicine. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530.
Assifi MM, Suchankova G, Constant S, Prentki M, Saha AK, Ruderman NB. AMP-activated protein kinase and coordination of hepatic fatty acid metabolism of starved/carbohydrate-refed rats. Am J Physiol Endocrinol Metab. 2005;289:E794–800.
Banz WJ, Maher MA, Thompson WG, Bassett DR, Moore W, Ashraf M, Keefer DJ, Zemel MB. Effects of resistance versus aerobic training on coronary artery disease risk factors. Exp Biol Med (Maywood). 2003;228:434–440.
Booth FW, Baldwin KM. Muscle plasticity: energy demand and supply processes. Rowell LB, Shepherd JT, editors. Exercise: regulation and integration of multiple systems. New York: Published for the American Physiological Society by Oxford University Press; 1996. p. 1075–1123.
Bouchard C, Malina RM, Perusse L. Genetics of fitness and physical performance. Champaign (IL): Human Kinetics; 1997.
Bouzakri K, Austin R, Rune A, Lassman ME, Garcia-Roves PM, Berger JP, Krook A, Chibalin AV, Zhang BB, Zierath JR. Malonyl CoenzymeA decarboxylase regulates lipid and glucose metabolism in human skeletal muscle. Diabetes. 2008;57:1508–1516.
Bradford LW. Problems of ethics and behavior in the forensic sciences. J Forensic Sci. 1976;21:763–768.
Braith RW, Stewart KJ. Resistance exercise training: its role in the prevention of cardiovascular disease. Circulation. 2006;113:2642–2650.
Brown MS, Goldstein JL. The SREBP pathway: regulation of cholesterol metabolism by proteolysis of a membrane-bound transcription factor. Cell. 1997;89:331–340.
Castaneda C, Layne JE, Munoz-Orians L, Gordon PL, Walsmith J, Foldvari M, Roubenoff R, Tucker KL, Nelson ME. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care. 2002;25:2335–2341.
Coon PJ, Bleecker ER, Drinkwater DT, Meyers DA, Goldberg AP. Effects of body composition and exercise capacity on glucose tolerance, insulin, and lipoprotein lipids in healthy older men: a cross-sectional and longitudinal intervention study. Metabolism. 1989;38:1201–1209.
Donnelly JE, Hill JO, Jacobsen DJ, Potteiger J, Sullivan DK, Johnson SL, Heelan K, Hise M, Fennessey PV, Sonko B, Sharp T, Jakicic JM, Blair SN, Tran ZV, Mayo M, Gibson C, Washburn RA. Effects of a 16-month randomized controlled exercise trial on body weight and composition in young, overweight men and women: the Midwest Exercise Trial. Arch Intern Med. 2003;163:1343–1350.
Edwards PA, Tabor D, Kast HR, Venkateswaran A. Regulation of gene expression by SREBP and SCAP. Biochim Biophys Acta. 2000;1529:103–113.
Elliott KJ, Sale C, Cable NT. Effects of resistance training and detraining on muscle strength and blood lipid profiles in postmenopausal women. Br J Sports Med. 2002;36:340–344.
Fahlman MM, Boardley D, Lambert CP, Flynn MG. Effects of endurance training and resistance training on plasma lipoprotein profiles in elderly women. J Gerontol A Biol Sci Med Sci. 2002;57:B54–60.
Foretz M, Carling D, Guichard C, Ferré P, Foufelle F. AMP-activated protein kinase inhibits the glucose-activated expression of fatty acid synthase gene in rat hepatocytes. J Biol Chem. 1998;273:14767–14771.
Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502.
Goodpaster BH, He J, Watkins S, Kelley DE. Skeletal muscle lipid content and insulin resistance: evidence for a paradox in endurance-trained athletes. J Clin Endocrinol Metab. 2001;86:5755–5761.
Guillet-Deniau I, Mieulet V, Le Lay S, Achouri Y, Carré D, Girard J, Foufelle F, Ferré P. Sterol regulatory element binding protein-1c expression and action in rat muscles: insulin-like effects on the control of glycolytic and lipogenic enzymes and UCP3 gene expression. Diabetes. 2002;51:1722–1728.
Hardie DG, Carling D. The AMP-activated protein kinase--fuel gauge of the mammalian cell? Eur J Biochem. 1997;246:259–273.
Hayashi T, Hirshman MF, Fujii N, Habinowski SA, Witters LA, Goodyear LJ. Metabolic stress and altered glucose transport: activation of AMP-activated protein kinase as a unifying coupling mechanism. Diabetes. 2000;49:527–531.
Ikeda S, Miyazaki H, Nakatani T, Kai Y, Kamei Y, Miura S, Tsuboyama-Kasaoka N, Ezaki O. Up-regulation of SREBP-1c and lipogenic genes in skeletal muscles after exercise training. Biochem Biophys Res Commun. 2002;296:395–400.
Irrcher I, Adhihetty PJ, Joseph AM, Ljubicic V, Hood DA. Regulation of mitochondrial biogenesis in muscle by endurance exercise. Sports Med. 2003;33:783–793.
Kelley GA, Kelley KS. Impact of progressive resistance training on lipids and lipoproteins in adults: a meta-analysis of randomized controlled trials. Prev Med. 2009;48:9–19.
Koves TR, Ussher JR, Noland RC, Slentz D, Mosedale M, Ilkayeva O, Bain J, Stevens R, Dyck JR, Newgard CB, Lopaschuk GD, Muoio DM. Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7:45–56.
Kuhl JE, Ruderman NB, Musi N, Goodyear LJ, Patti ME, Crunkhorn S, Dronamraju D, Thorell A, Nygren J, Ljungkvist O, Degerblad M, Stahle A, Brismar TB, Andersen KL, Saha AK, Efendic S, Bavenholm PN. Exercise training decreases the concentration of malonyl-CoA and increases the expression and activity of malonyl-CoA decarboxylase in human muscle. Am J Physiol Endocrinol Metab. 2006;290:E1296–1303.
Lee S, Barton ER, Sweeney HL, Farrar RP. Viral expression of insulin-like growth factor-I enhances muscle hypertrophy in resistance-trained rats. J Appl Physiol (1985). 2004;96:1097–1104.
Lima RM, Bezerra LM, Rabelo HT, Silva MA, Silva AJ, Bottaro M, de Oliveira RJ. Fat-free mass, strength, and sarcopenia are related to bone mineral density in older women. J Clin Densitom. 2009;12:35–41.
Lokey EA, Tran ZV. Effects of exercise training on serum lipid and lipoprotein concentrations in women: a meta-analysis. Int J Sports Med. 1989;10:424–429.
McGarry JD, Brown NF. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur J Biochem. 1997;244:1–14.
Melanson EL, Ingebrigtsen JP, Bergouignan A, Ohkawara K, Kohrt WM, Lighton JR. A new approach for flow-through respirometry measurements in humans. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1571–1579.
Merrill GF, Kurth EJ, Hardie DG, Winder WW. AICA riboside increases AMP-activated protein kinase, fatty acid oxidation, and glucose uptake in rat muscle. Am J Physiol. 1997;273:6 Pt 1. E1107–1112.
Miyazaki M, Kim YC, Ntambi JM. A lipogenic diet in mice with a disruption of the stearoyl-CoA desaturase 1 gene reveals a stringent requirement of endogenous monounsatu-rated fatty acids for triglyceride synthesis. J Lipid Res. 2001;42:1018–1024.
Muoio DM, Seefeld K, Witters LA, Coleman RA. AMP-activated kinase reciprocally regulates triacylglycerol synthesis and fatty acid oxidation in liver and muscle: evidence that sn-glycerol-3-phosphate acyltransferase is a novel target. Biochem J. 1999;338:Pt 3. 783–791.
Nadeau KJ, Ehlers LB, Aguirre LE, Moore RL, Jew KN, Ortmeyer HK, Hansen BC, Reusch JE, Draznin B. Exercise training and calorie restriction increase SREBP-1 expression and intramuscular triglyceride in skeletal muscle. Am J Physiol Endocrinol Metab. 2006;291:E90–98.
Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577.
Poirier P, Catellier C, Tremblay A, Nadeau A. Role of body fat loss in the exercise-induced improvement of the plasma lipid profile in non-insulin-dependent diabetes mellitus. Metabolism. 1996;45:1383–1387.
Rabelo HT, Bezerra LA, Terra DF, Lima RM, Silva MA, Leite TK, de Oliveira RJ. Effects of 24 weeks of progressive resistance training on knee extensors peak torque and fat-free mass in older women. J Strength Cond Res. 2011;25:2298–2303.
Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, Hailpern SM, Ho M, Howard V, Kissela B, Kittner S, Lloyd-Jones D, McDermott M, Meigs J, Moy C, Nichol G, O’Donnell C, Roger V, Sorlie P, Steinberger J, Thom T, Wilson M, Hong Y. American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146.
Ruderman NB, Saha AK, Kraegen EW. Minireview: malonyl CoA, AMP-activated protein kinase, and adiposity. Endocrinology. 2003;144:5166–5171.
Russell AP, Hesselink MK, Lo SK, Schrauwen P. Regulation of metabolic transcriptional co-activators and transcription factors with acute exercise. FASEB J. 2005;19:986–988.
Saha AK, Schwarsin AJ, Roduit R, Masse F, Kaushik V, Tornheim K, Prentki M, Ruderman NB. Activation of malonyl-CoA decarboxylase in rat skeletal muscle by contraction and the AMP-activated protein kinase activator 5-aminoimidazole-4-carboxamide-1-beta -D-ribofuranoside. J Biol Chem. 2000;275:24279–24283.
Sakakura Y, Shimano H, Sone H, Takahashi A, Inoue N, Toyoshima H, Suzuki S, Yamada N. Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem Biophys Res Commun. 2001;286:176–183.
Sakamoto K, Goodyear LJ. Invited review: intracellular signaling in contracting skeletal muscle. J Appl Physiol (1985). 2002;93:369–383.
Schrauwen P, Lichtenbelt WD, Saris WH, Westerterp KR. Fat balance in obese subjects: role of glycogen stores. Am J Physiol. 1998;274:6 Pt 1. E1027–1033.
Trevisan MC, Souza JM, Marucci Mde F. Influence of soy protein intake and weight training on the resting energy expenditure of postmenopausal women. Rev Assoc Med Bras. 2010;56:572–578.
Vincent KR, Braith RW, Bottiglieri T, Vincent HK, Lowenthal DT. Homocysteine and lipoprotein levels following resistance training in older adults. Prev Cardiol. 2003;6:197–203.
Warburton DE, Gledhill N, Quinney A. Musculoskeletal fitness and health. Can J Appl Physiol. 2001;26:217–237.
Washburn RA, Kirk EP, Smith BK, Honas JJ, Lecheminant JD, Bailey BW, Donnelly JE. One set resistance training: effect on body composition in overweight young adults. J Sports Med Phys Fitness. 2012;52:273–279.
Woods A, Azzout-Marniche D, Foretz M, Stein SC, Lemarchand P, Ferré P, Foufelle F, Carling D. Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol Cell Biol. 2000;20:6704–6711.
Fig. 1The activity of lipogenesis genes, measured as mRNA expression, in the biceps muscle tissue of rats after 12 weeks of resistance training. Values are presented as mean±standard deviation and presented as the absolute value of mRNA volume. CON, control (rest) group; RT, resistance training group (n=6 for each group); SREBP-Ic, sterol regulatory element binding protein-1c; FAS, fatty acid synthase; ACC, acetyl-CoA carboxylase. 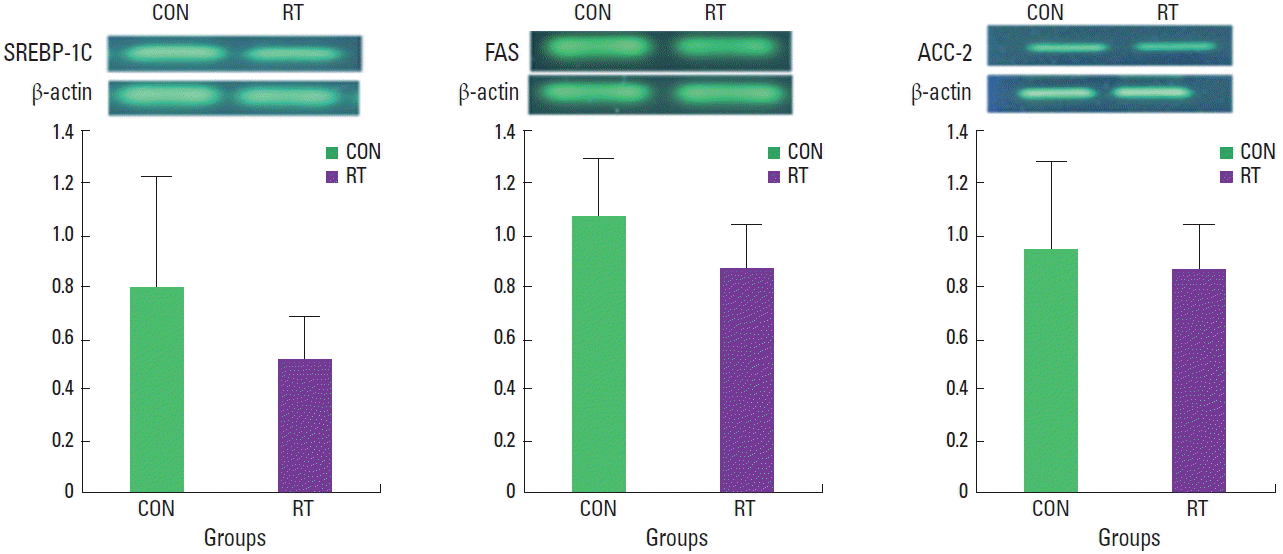
Fig. 2The activity of lipolysis genes, measured as mRNA expression, in the biceps muscle tissue of rats after 12 weeks of resistance training. Values are presented as mean±standard deviation and presented as the absolute value of mRNA volume. CON, control (rest) group; RT, resistance training group (n=6 for each group). LPL, lipoprotein lipase; CPT-1, carnitine palmitoyl transferase 1; MCD, malonyl-CoA decarboxylase. *P<0.05. ***P<0.001. 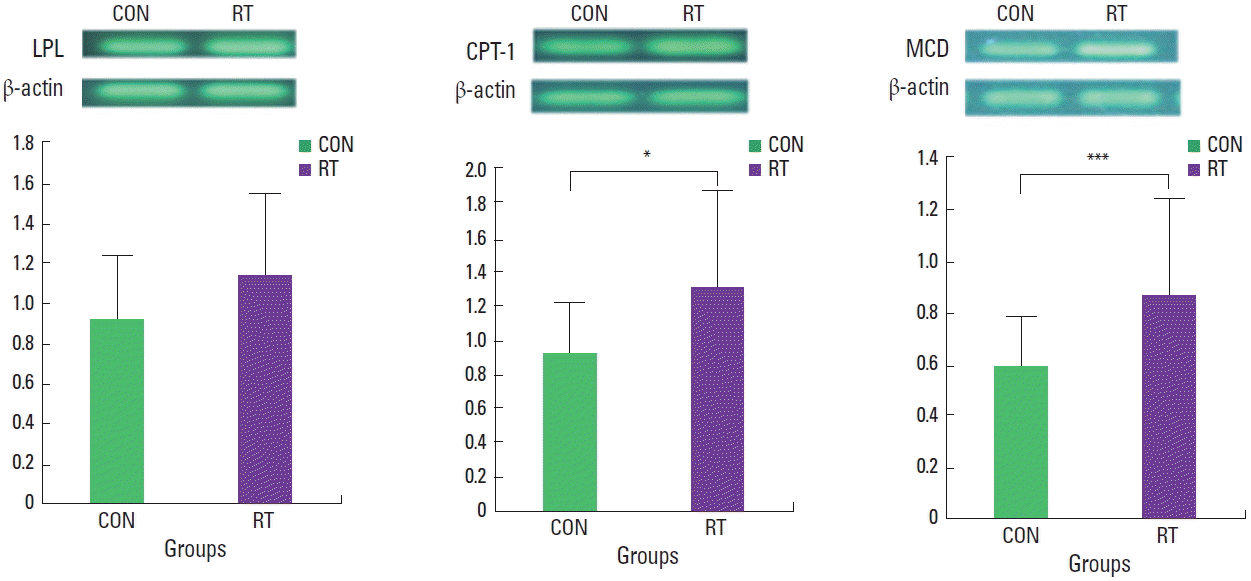
Fig. 3AMP-activated protein kinase (AMPK) protein levels in the biceps muscle tissue of rats after 12 weeks of resistance training. Values are presented as mean±standard deviation and presented as the absolute value of protein volume. CON, control (rest) group; RT, resistance training group (n=6 for each group). ***P<0.001. 
Table 1Changes in the body weight of rats during 12 weeks of resistance training
Table 2Blood lipid profiles of rats during 12 weeks of resistance training
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||