AbstractDuring pregnancy, diabetes mellitus exerts detrimental effects on the development of the fetus, especially the central nervous system. In the current study, we evaluated the effects of postnatal treadmill exercise on short-term memory in relation with cell proliferation and apoptosis in the hippocampus of rat pups born to streptozotocin (STZ)-induced diabetic maternal rats. Adult female rats were mated with male rats for 24 h. Two weeks after mating, the pregnant female rats were divided into two groups: control group and STZ injection group. The pregnant rats in the STZ injection group were administered 40 mg/kg of STZ intraperitoneally. After birth, the rat pups were divided into the following four groups: control group, control with postnatal exercise group, maternal STZ-injection group, and maternal STZ-injection with postnatal exercise group. The rat pups in the postnatal exercise groups were made to run on a treadmill for 30 min once a day, 5 times per week for 2 weeks beginning 4 weeks after birth. The rat pups born to diabetic rats were shown to have short-term memory impairment with suppressed cell proliferation and increased apoptosis in the hippocampal dentate gyrus. Postnatal treadmill exercise alleviated short-term memory impairment by increased cell proliferation and suppressed apoptosis in the rat pups born to diabetic rats. These findings indicate that postnatal treadmill exercise may be used as a valuable strategy to ameliorate neurodevelopmental problems in children born to diabetics.
INTRODUCTIONDiabetes mellitus is known to exert detrimental effects on the development of the fetus during pregnancy. These effects primarily occur in the central nervous system (CNS) and are associated with long-term neurologic dysfunction, such as deficits in attention, cognitive function, motor control, and perception during childhood (Aberg and Westbom, 2001). In addition, there is abundant epidemiologic and experimental evidence demonstrating a close correlation between the degree of maternal glycemic control and the intelligence and behavior of infants born to diabetic mothers (Suh et al., 2005; van Assche et al., 2001). Furthermore, the fetal environment during a pregnancy complicated by diabetes is characterized by chronic hypoxia and iron deficiency, accompanied by intermittent acute changes in glucose status and acidemia (Nelson et al., 2000). All of these conditions can cause damage to the development of the fetal brain, particularly the hippocampus (Nelson et al., 2000; Raman et al., 2005).
The hippocampal formation is a region of the brain implicated in learning and memory acquisition. New cells are produced continuously in the hippocampal dentate gyrus of mammals, including humans, during development and throughout adulthood (Drapeau et al., 2003; Kempermann et al., 1997). Hippocampal neurogenesis can be influenced by several environmental factors and stimuli (Brown et al., 2003; Kempermann et al., 1997), and adverse prenatal environmental conditions can suppress formation of hippocampal granule cells (Coe et al., 2003; Kim et al., 2006). Running on a wheel or on a treadmill increases cell proliferation and/or neurogenesis in the dentate gyrus of the rodent hippocampus (Brown et al., 2003; Kim et al., 2003; Trejo et al., 2001).
The term apoptosis was coined by Kerr et al. (Kerr et al., 1972). Apoptosis is a form of cell death that involves scattered discrete cells rather than a confluent volume of tissues. Apoptosis is characterized by a series of distinctive morphologic changes, including shrinkage of cells, marked condensation of chromatin, formation of cytoplasmic protuberances on the surface of cells, and fragmentation of cells by separation of the protuberances to form multiple small membrane-bound bodies that contain intact organelles and/or dense clumps of condensed chromatin (Wyllie et al., 1981). Apoptotic cell death can be assessed by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining, which detects DNA fragmentation (Kim et al., 2010). In addition, another important characteristic of apoptotsis is the activation of caspase-3, which is a family of cysteinyl proteases and one of the key mediators of apoptosis in mammalian cells (Kim et al., 2010). Bax is a pro-apoptotic member of the Bcl-2 protein family that is predominantly localized in the cytosol of healthy cells, and translocates to the mitochondria after a variety of death stimuli (Cory and Adams, 2002). Once Bax translocates to the mitochondria where it forms homo- and hetero-dimers or clusters, interacts with other members of the Bcl-2 family, causes loss of mitochondrial membrane potential, and finally induces apoptotic cell death (Antonsson et al., 2001).
Physical exercise is known to improve cognitive function, memory, and learning, as well as to decrease the risks of cognitive impairment induced by various brain insults (Cotman and Berchtold, 2002; Heo et al., 2014; Jeong et al., 2014; Kim et al., 2010; Reisi et al., 2009). Exercise also up-regulates the production of neuropeptides and neurotrophins, such as neurotensin, substance P, and brain-derived neurotrophic factor (BDNF) mRNA in the rat hippocampus. These neurotransmitters are implicated in neuronal survival, differentiation, connectivity, and synaptic plasticity (Chen et al., 1999; Kim et al., 2014). Exercise is also known as a useful intervention for the treatment of diabetes mellitus. Regular physical exercise is effective for preventing and delaying the onset of symptoms and complications of diabetes, and exercise also increases insulin sensitivity and ameliorates disturbances in glucose metabolism (Derouich and Boutayeb, 2002). Physical exercise increases blood flow, enhances glucose uptake in muscles, reduces the level of triglycerides, and improves endothelial function (Chakraphan et al., 2005). Insulin resistance and glucose-stimulated insulin secretion play important roles in regulating glucose homeostasis (Kaastra et al., 2006).
It is well-established that treadmill exercise is helpful for the diabetic patients; however, the effects of postnatal treadmill exercise on brain function of the offspring born to diabetics. In the current study we evaluated the effects of postnatal treadmill exercise on memory ability, cell proliferation, and apoptotic neuronal cell death in the hippocampal dentate gyrus of rat pups born to diabetic rats. For the current study a step-down avoidance task, TUNEL assay, and immunohistochemistry for 5-bromo-2′-deoxyuridine (BrdU), caspase-3, and Bax were performed.
MATERIALS AND METHODSAnimals and treatmentsIn the present study adult female Sprague-Dawley rats weighing 220±10 g (n=40) and adult male Sprague-Dawley rats weighing 300±10 g (n=40) were mated for 24 h. After mating, female rats were housed individually in a plastic home cage at a controlled temperature (20±2°C) and maintained under a light-dark cycle consisting of 12 h of light and 12 h of darkness (lights on from 07:00 h to 19:00 h), with food and water made available ad libitum. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences.
Pregnant rats were randomly divided into 2 groups: control and streptozotocin (STZ)-injection groups (n=12 in each group). On day 7 of gestation, the pregnant rats in the STZ injection group were administered 40 mg/kg of STZ intraperitoneally (Sigma Chemical Co., St. Louis, MO, USA) in 40 mM citrate buffer (pH 4.5). To determine the blood glucose concentrations of maternal rats, 50 μg blood samples were collected from the tail vein 2 days after STZ injection, and concentrations of glucose were measured using a blood glucose tester (Arkray, Kyoto, Japan). The animals with blood glucose levels>300 mg/dL were used as the diabetic group. The pregnant rats in the control group were administered an equal volume of normal saline intraperitoneally. After birth, the rat pups in each group were left undisturbed together with their mothers for 28 days. The rat pups were then divided into the following four groups: control group; control with postnatal exercise group; maternal STZ-injection group; and maternal STZ-injection with postnatal exercise group (n=12 in each group).
Exercise protocolsThe rat pups in the postnatal exercise groups performed treadmill running for 2 weeks starting 4 weeks after birth. Treadmill exercising continued for 30 min once a day 5 times per week for 2 weeks. The workload of the exercise consisted of running at a speed of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min, with a zero degree inclination. The rat pups in the non-exercise groups were left on the treadmill without running for 30 min for the same duration of time. All rat pups were injected with 50 mg/kg of 5-bromo-2′-deoxyuridine (BrdU) intraperitoneally 1 h prior to the beginning of each exercise session.
Determination of short-term memoryShort-term memory was evaluated by assessing the latency of the step-down avoidance task, according to the previously described method (Heo et al., 2014). The rat pups were trained in the step-down avoidance task. In brief, the rat pups were placed on a 7×25 cm platform at a height of 2.5 cm, then allowed to rest for 2 min. The platform faced a 42×25 cm grid of parallel 0.1-cm caliber stainless steel bars that were spaced 1 cm apart. During the training session on the 12 days after starting treadmill exercise (postnatal day 40), the animals received a 0.5 mA scramble foot shock for 2 sec immediately upon stepping down. The retention time was then determined 2 days after the training session (post-natal day 42). The latency of the step-down avoidance task was defined as a rat pup stepping down and placing all four paws on the grid. Latencies>300 sec were counted as 300 sec.
Tissue preparationImmediately after determination of the retention time, the rat pups were anesthetized using Zoletil 50® (10 mg/kg, ip; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM of phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB; pH 7.4). The brains were dissected and post-fixed in the same fixative overnight, and transferred to a 30% sucrose solution for cryoprotection. Coronal sections, 40-μm thick, were made with a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistryIn order to detect newly generated cells in the dentate gyrus, BrdU-specific immunohistochemistry was performed according to a previously described method (Kim et al., 2014). The sections were permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, after which the sections were pre-treated with 50% formamide-2X standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, then rinsed twice in 100 mM sodium borate (pH 8.5). Next, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed 3 times with PBS and incubated for 1 h with a biotinylated secondary murine antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were then incubated for an additional h using the VECTASTAIN® Elite ABC Kit (1:100; Vector Laboratories) according to the manufacturer’s instructions. For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.02% 3,3′-diaminobenzidine (DAB), nickel chloride (40 mg/mL), and 0.03% hydrogen peroxide for 5 min.
After BrdU-specific staining, counter-staining was performed on the same sections using mouse anti-neuronal nucleic (NeuN) antibody (1:300; Chemicon International, Temecula, CA, USA). The sections were washed 3 times with PBS and incubated for 1 h with a biotinylated secondary anti-mouse antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.02% DAB and 0.03% hydrogen peroxide for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific, Fair Lawn, NJ, USA).
Caspase-3 immunohistochemistryFor the visualization of the caspase-3 expression, caspase-3 immunohistochemistry was performed as a previously described method (Choi et al., 2013). In brief, the sections were incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Bio-technology Inc., Santa Cruz, CA, USA), then for another h with the biotinylated secondary murine antibody. The bound secondary antibody was then amplified with a Vector Elite ABC kit®. The antibody-biotin-avidin-peroxidase complex was visualized using 0.02% DAB. The sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Bax immunohistochemistryBax immunohistochemistry was performed according to a previously described method (Yang et al., 2010). The sections were drawn from each brain and incubated overnight with mouse anti-Bax antibody (1:500; Santa Cruz Biotechnology Inc.). The sections were then washed 3 times with PBS and incubated for 1 h with a biotinylated secondary murine antibody (1:200; Vector Laboratories). The bound secondary antibody was then amplified with a Vector Elite ABC kit®. The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
TUNEL stainingThe TUNEL assay was performed using an in situ Cell Death Detection Kit® (Roche) according to the manufacturer’s protocol (Choi et al., 2013). The sections were fixed in ethanol-acetic acid (2:1) and rinsed. The sections were then incubated with 100 μg/mL proteinase K, rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.02% DAB. Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used for counter-staining and the sections were finally mounted onto gelatin-coated slides. The slides were air-dried overnight at room temperature, and covers-lips were mounted with Permount® (Fisher Scientific).
Data analysisThe numbers of BrdU-positive, caspase-3-positive, Bax-positive, and TUNEL-positive cells in the hippocampal dentate gyrus were counted hemi-laterally using Image-Pro® Plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). These data are expressed as the number of cells per square millimeter (mm2) of cross-sectional area in each of the selected hippocampal dentate gyri. All values are expressed as the mean±standard error of the mean (SEM). For comparisons among the groups, one-way analysis of variance (ANOVA) and Duncan’s post-hoc test were performed with a P<0.05 as an indication of statistical significance.
RESULTSEffect of postnatal treadmill exercise on memory in the step-down avoidance taskThe latency of the step-down avoidance task was 126.25±12.31 sec in the control group, 156.91±14.05 sec in the control with postnatal exercise group, 66.16±15.45 sec in the maternal STZ-injection group, and 115.66±17.68 sec in the maternal STZ-injection with postnatal exercise group (Fig. 1). These findings indicate that the rat pups born to the diabetic rats had significant memory retardation, and the postnatal treadmill exercise ameliorated the memory impairment (P<0.05). In the normal rat pups, the postnatal treadmill exercise also enhanced memory function (P< 0.05).
Effect of postnatal treadmill exercise on the number of BrdU-positive cells in the hippocampal dentate gyrusThe number of BrdU-positive cells in the hippocampal dentate gyrus was 1,214.09±75.11/mm2 in the control group, 1,418.52± 84.8/mm2 in the control with postnatal exercise group, 862.87± 81.39/mm2 in the maternal STZ-injection group, and 1,149.23± 92.60/mm2 in the maternal STZ-injection with postnatal exercise group (Fig. 2). The results indicate that the rat pups born to the diabetic rats had a significant decrease in cell proliferation in the hippocampal dentate gyrus (P<0.05), and the postnatal treadmill exercise increased cell proliferation (P<0.05). In normal rat pups, postnatal treadmill exercise also enhanced cell proliferation (P<0.05).
Effect of postnatal treadmill exercise on the number of caspase-3-positive cells in the hippocampal dentate gyrusPhotomicrographs of the caspase-3-positive cells in the hippocampal dentate gyrus are presented in Fig. 3. The number of caspase-3-positive cells was 23.65±7.40/mm2 in the control group, 16.91±4.80/mm2 in the control with postnatal exercise group, 112.38±12.82/mm2 in the maternal STZ-injection group, and 74.51±12.12/mm2 in the maternal STZ-injection with postnatal exercise group (Fig. 3). The results indicate that the rat pups born to the diabetic rats had a significant increase in caspase-3 expression in the hippocampal dentate gyrus (P<0.05), and postnatal treadmill exercise inhibited caspase-3 expression (P<0.05). In normal rat pups, postnatal treadmill exercise exerted no significant effect on caspase-3 expression.
Effect of postnatal treadmill exercise on the number of Bax-positive cells in the hippocampal dentate gyrusPhotomicrographs of Bax-positive cells in the hippocampal dentate gyrus are presented in Fig. 4. The number of Bax-positive cells was 22.87±4.22/mm2 in the control group, 30.61±6.54/mm2 in the control with postnatal exercise group, 125.71±7.61/mm2 in the maternal STZ-injection group, and 79.16±8.33/mm2 in the maternal STZ-injection with postnatal exercise group (Fig. 4). The results indicate that rat pups born to diabetic rats had a significant increase in Bax expression in the hippocampal dentate gyrus (P<0.05), and postnatal treadmill exercise inhibited Bax expression (P<0.05). In normal rat pups, postnatal treadmill exercise exerted no significant effect on Bax expression.
Effect of postnatal treadmill exercise on the number of TUNEL-positive cells in the hippocampal dentate gyrusPhotomicrographs of the TUNEL-positive cells in the hippocampal dentate gyrus are presented in Fig. 5. The number of TUNEL-positive cells was 7.58±2.19/mm2 in the control group, 4.85 ±1.34/mm2 in the control with postnatal exercise group, 34.06± 3.31/mm2 in the maternal STZ-injection group, and 14.71±2.56 /mm2 in the maternal STZ-injection with postnatal exercise group (Fig. 5). The results indicate that rat pups born to diabetic rats had a significant increase in DNA fragmentation in the hippocampal dentate gyrus (P<0.05) and postnatal treadmill exercise inhibited DNA fragmentation (P<0.05). In normal rat pups, post-natal treadmill exercise exerted no significant effect on apoptosis.
DISCUSSIONPregnancy in type 1 diabetics is characterized by very wide fluctuations in 24 h glycemia with high postprandial peaks. A single high-dose of STZ exerts irreversible damage to insulin-producing cells, therefore STZ has been extensively used to induce insulin-dependent (type 1) diabetes in animals (Kim et al., 2003). Maternal diabetes during pregnancy causes memory deficits in a hippocampal-dependent task of offspring (DeBoer et al., 2005). It has been suggested that infants of diabetic mothers experienced perturbation in memory performance due to exposure to multiple neurologic risk factors, including chronic hypoxia, hyperglycemia/reactive hypoglycemia, and iron deficiency (DeBoer et al., 2005). In the present study, the rat pups born to diabetic rats had delayed latency in the step-down avoidance task, suggesting memory impairment.
Exercise is known to exert an ameliorative effect on cognitive impairment and dementia in elderly persons, and exercise also exerts a protective effect against brain damage induced by various pathologic conditions (Griesbach et al., 2004; Heo et al., 2014; Jeong et al., 2014; Kim et al., 2010). Furthermore, exercise reversed the nutrition-mediated reduction in learning ability (Molteni et al., 2004). In the present study, postnatal treadmill exercise alleviated memory impairment in rat pups born to diabetic rats. Postnatal treadmill exercise also enhanced memory ability in rat pups born to normal rats.
Various prenatal stresses decrease cell proliferation in the hippocampal dentate gyrus of offspring (Coe et al., 2003; Kim et al., 2006). The intrauterine conditions of diabetic mothers are known to exert detrimental effects on the fetal hippocampus (DeBoer et al., 2005; Suh et al., 2005). In the present study, cell proliferation in the hippocampal dentate gyrus was significantly suppressed in rat pups born to diabetic rats.
In mammals, including humans, neuronal precursors reside in the subgranular zone of the dentate gyrus and they proliferate and migrate continuously into the granule cell layer, then differentiate into mature neurons with morphologic and biochemical features similar to those of the surrounding neurons (Kempermann et al., 1997). Schmidt-Hieber et al. (2004) suggested that these newly generated neurons in the adult brain are essential for the formation of new memories. Increased cell proliferation in the hippocampal dentate gyrus of rats improves learning ability (Kempermann et al., 1997; Kim et al., 2014; Sim et al., 2004), while stress and aging reduce cell proliferation, resulting in impairment of learning ability and memory function (Coe et al., 2003; Drapeau et al., 2003). It has been established that exercise enhances cell proliferation and/or neurogenesis in the hippocampus (Kim et al., 2003; Sim et al., 2004; Trejo et al., 2001). In the present study, postnatal treadmill running enhanced cell proliferation in the hippocampal dentate gyrus of rat pups born to diabetic rats. Postnatal treadmill exercise also increased cell proliferation in the hippocampal dentate gyrus of rat pups born to normal rats.
Apoptosis assessed by TUNEL staining and caspase-3 immunoreactivity was increased in the sensorimotor cortex and the hippocampus in diabetic rats (Rizk et al., 2007). After 8 weeks of poorly-controlled diabetes, the prototypic caspases of the extrinsic cell death pathway were activated (Wajant, 2002), possibly explaining the increased death rate. Kuhad et al. (2009) reported that diabetes increased caspase-3 activity in the cerebral cortex and hippocampus. In the present study, the numbers of caspase-3-positive, Bax-positive, and TUNEL-positive cells were increased in rat pups born to diabetic rats.
Sim et al. (2004) reported that treadmill exercise significantly suppresses ischemia-induced increase in DNA fragmentation and caspase-3 expression in the hippocampus, and thus facilitates the recovery of short-term memory impairment. Treadmill exercise suppresses apoptosis in the hippocampus of STZ-induced diabetic rats (Lee et al., 2005). Ghosh et al. (2009) reported that moderate exercise reduces caspase-3 and caspase-8 activities in diabetic mice. Williamson et al. (2010) reported that diabetic cardiac mitochondria apoptosis was attenuated in exercised animals, as indicated by Bax expression and caspase-3 activity. A reduction in caspase-3 and caspase-8 activities induced by exercise is related with reduced oxidative damage, and underlies the beneficial effects of exercise in diabetic mice (Ghosh et al., 2009). In the present study, postnatal treadmill exercise suppressed the number of caspase-3-positive, Bax-positive, and TUNEL-positive cells in the hippocampal dentate gyrus of rat pups born to diabetic rats, demonstrating that postnatal treadmill exercise alleviated diabetic-induced apoptotic neuronal cell death in rat pups.
In this study, rat pups born to diabetic rats had impaired memory function with decreased cell proliferation and increased apoptosis in the hippocampus. Postnatal treadmill exercise alleviated memory impairment with enhanced cell proliferation and suppressed apoptosis in the hippocampus of rat pups born to diabetic rats. The results of the present study indicate that treadmill exercise may be used as a valuable strategy to ameliorate neurodevelopmental problems in children born to diabetics during pregnancy.
ACKNOWLEDGMENTSThis work was supported by the National Research Foundation of Korea Grant funded by the Korean Government (NRF-2009-352-G00056).
REFERENCESAberg A, Westbom L. Association between maternal pre-existing or gestational diabetes and health problems in children. Acta Paediatr. 2001;90:746–750.
Antonsson B, Montessuit S, Sanchez B, Martinou JC. Bax is presents as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem. 2001;276:1615–1623.
Brown J, Cooper-Kuhn C, Kempermann G, Van Praag H, Winkler J, Gage F, Kuhn HG. Enrichd environment and physical activity stimulate hippocampal but not olfactory bulb neurogenesis. Eur J Neurosci. 2003;17:2042–2046.
Chakraphan D, Sridulyakul P, Thipakorn B, Bunnag S, Huxley VH, Patumraj S. Attenuation of endothelial dysfunction of exercise training in STZ-induced diabetic rats. Clin Hemorheol Microcirc. 2005;23:217–226.
Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J Neurosci. 1999;19:7983–7990.
Choi JH, Kim TS, Park JK, Sim YJ, Kim K, Lee SJ. Short-term treadmill exercise preserves sensory-motor function through inhibiting apoptosis in the hippocampus of hypoxic ischemia injury rat pups. J Exerc Rehabil. 2013;9:457–462.
Coe CL, Kramer M, Czéh B, Gould E, Reeves AJ, Kirschbaum C, Fuchs E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034.
Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev. 2002;2:647–656.
Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301.
DeBoer T, Wewerka S, Bauer PJ, Georgieff MK, Nelson CA. Explicit memory performance in infants of diabetic mothers at 1 year of age. Dev Med Child Neurol. 2005;47:525–531.
Derouich M, Boutayeb A. The effect of physical exercise on the dynamics of glucose and insulin. J Biomech. 2002;35:911–917.
Drapeau E, Mayo W, Aurousseau C, Le Moal M, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2003;100:14385–14390.
Ghosh S, Khazaei M, Moien-Afshari F, Ang LS, Granville DJ, Verchere CB, et al. Moderate exercise attenuates caspase-3 activity, oxidative stress, and inhibits progression of diabetic renal disease in db/db mice. J Physiol Renal Physiol. 2009;296:F700–708.
Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139.
Heo YM, Shin MS, Lee JM, Kim CJ, Baek SB, Kim KH, Baek SS. Treadmill exercise ameliorates short-term memory disturbance in scopolamine-induced amnesia rats. Int Neurourol J. 2014;18:16–22.
Jeong HI, Ji ES, Kim SH, Kim TW, Baek SB, Choi SW. Treadmill exercise improves spatial learning ability by enhancing brain-derived neurotrophic factor expression in the attention-deficit/hyperactivity disorder rats. J Exerc Rehabil. 2014;10:162–167.
Kaastra B, Manders RJ, Van Breda E, Kies A, Jeukendrup AE, Keizer HA, Kuipers H, Van Loon LJ. Effects of increasing insulin secretion on acute postexercise blood glucose disposal. Med Sci Sports Exerc. 2006;38:268–275.
Kempermann G, Kuhn HG, Gage FH. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386:493–495.
Kerr JF, Wyllie AH, Currie AR. Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer. 1972;26:239–257.
Kim BK, Shin MS, Kim CJ, Baek SB, Ko YC, Kim YP. Treadmill exercise improves short-term memory by enhancing neurogenesis in amyloid beta-induced Alzheimer disease rats. J Exerc Rehabil. 2014;10:2–8.
Kim DH, Ko IG, Kim BK, Kim TW, Kim SE, Shin MS, Kim CJ, Kim H, Kim KM, Baek SS. Treadmill exercise inhibits traumatic brain injury-induced hippocampal apoptosis. Physiol Behav. 2010;101:660–665.
Kim H, Lee MH, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, Won R, Shin HS, Kim CJ. Influence of prenatal noise and music on the spatial memory and neurogenesis in the hippocampus of developing rats. Brain Dev. 2006;28:109–114.
Kim HB, Jang MH, Shin MC, Lim BV, Kim YP, Kim KJ, Kim EH, Kim CJ. Treadmill exercise increases cell proliferation in dentate gyrus of rats with streptozotocin-induced diabetes. J Diabetes Complications. 2003;17:29–33.
Kuhad A, Bishnoi M, Tiwari V, Chopra K. Suppression of NF-κβ signaling pathway by tocotrienol can prevent diabetes associated cognitive deficits. Pharmacol Biochem Behav. 2009;92:251–259.
Lee HH, Shin MS, Kim YS, Yang HY, Chang HK, Lee TH, Kim CJ, Cho S, Hong SP. Early treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death and increases cell proliferation in the dentate gyrus of streptozotocin-induced hyperglycemic rats. J Diabetes Complications. 2005;19:339–346.
Molteni R, Wu A, Vaynman S, Ying Z, Barnard RJ, Gómez-Pinilla F. Exercise reverses the harmful effects of consumption of a high-fat diet on synaptic and behavioral plasticity associated to the action of brain-derived neurotrophic factor. Neuroscience. 2004;123:429–440.
Nelson CA, Wewerka S, Thomas KM, Tribby-Walbridge S, deRegnier R, Georgieff M. Neurocognitive sequelae of infants of diabetic mothers. Behav Neurosci. 2000;114:950–956.
Raman L, Tkac I, Ennis K, Georgieff MK, Gruetter R, Rao R. In vivo effect of chronic hypoxia on the neurochemical profile of the developing rat hippocampus. Brain Res. 2005;156:202–209.
Reisi P, Alaei H, Babri S, Sharifi MR, Mohaddes G. Effects of treadmill running on spatial learning and memory in streptozotocin-induced diabetic rats. Neurosci Lett. 2009;455:79–83.
Rizk NN, Myatt-Jones J, Rafols J, Dunbar JC. Insulin like growth factor-1 (IGF-1) decreases ischemia-reperfusion induced apoptosis and necrosis in diabetic rats. Endocrine. 2007;31:66–71.
Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187.
Sim YJ, Kim SS, Kim JY, Shin MS, Kim CJ. Treadmill exercise improves short-term memory by suppressing ischemia-induced apoptosis of neuronal cells in gerbils. Neurosci Lett. 2004;372:256–261.
Suh SW, Fan Y, Hong SM, Liu Z, Matsumori Y, Weinstein PR, et al. Hypoglycemia induces transient neurogenesis and subsequent progenitor cell loss in the rat hippocampus. Diabetes. 2005;54:500–509.
Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult. J Neurosci. 2001;21:1628–1634.
van Assche FA, Holemans K, Aerts L. Long-term consequences for off-spring of diabetes during pregnancy. Br Med Bull. 2001;60:173–182.
Fig. 1.Effect of postnatal treadmill exercise on latency in the step-down avoidance task of the rat pups. (A) Control group, (B) control with postnatal exercise group, (C) maternal streptozotocin (STZ)-injection group, and (D) maternal STZ-injection with postnatal exercise group. Values shown are the mean± SEM. *P< 0.05 when compared to the control group. #P< 0.05 when compared to the maternal STZ-injection group. 
Fig. 2.Effect of postnatal treadmill exercise on cell proliferation in the hippocampal dentate gyrus of the rat pups. Upper: Photomicrographs of 5-bromo-2’-deoxyuridine (BrdU)-positive cells in the dentate gyrus of the hippocampus. The sections were stained with BrdU (black) to identify newly generated cells and with mouse anti-neuronal nuclei antibody to determine the presence of neuronal nuclei (NeuN; brown). The scale bar represents 250 μm. Lower: The number of BrdU-positive cells in the dentate gyrus of the hippocampus. (A) Control group, (B) control with postnatal exercise group, (C) maternal streptozotocin (STZ)-injection group, and (D) maternal STZ-injection with postnatal exercise group. Values shown are the mean± SEM. *P< 0.05 when compared to the control group. #P< 0.05 when compared to the maternal STZ-injection group. 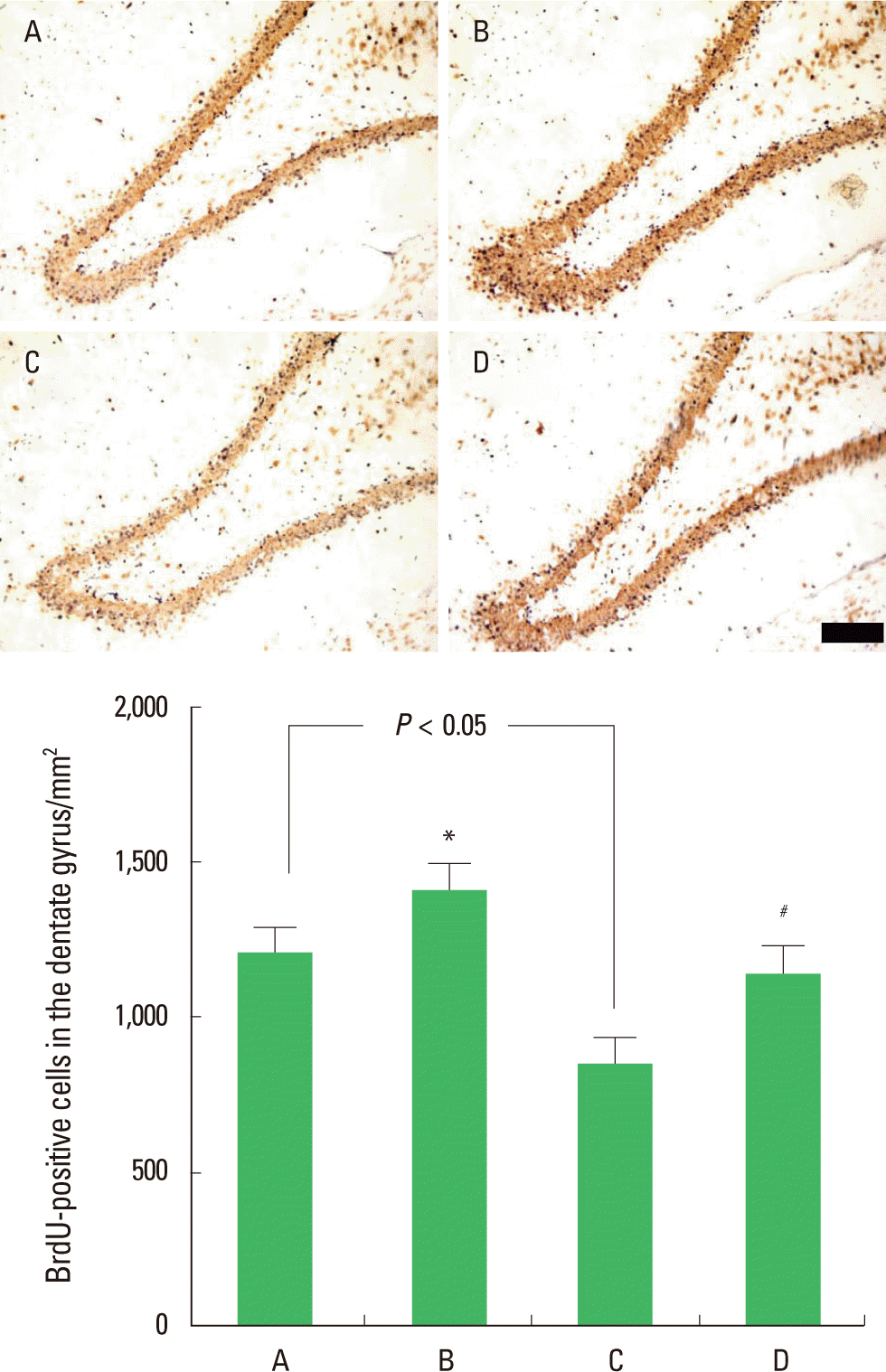
Fig. 3.Effect of postnatal treadmill exercise on caspase-3 expression in the hippocampal dentate gyrus of the rat pups. Upper: Photomicrographs showing immunostaining for caspase-3 in the dentate gyrus of the hippocampus. The scale bar represents 100 μm. Lower: The number of caspase-3-positive cells in each group. (A) Control group, (B) control with postnatal exercise group, (C) maternal streptozotocin (STZ)-injection group, and (D) maternal STZ-injection with postnatal exercise group. Values shown are the mean± SEM. *P< 0.05 when compared to the control group. #P< 0.05 when compared to the maternal STZ-injection group. 
Fig. 4.Effect of postnatal treadmill exercise on the number of Bax-positive cells in the hippocampal dentate gyrus of rat pups. Upper: Photomicrograph showing immunostaining for Bax in the dentate gyrus of the hippocampus. The scale bar represents 100 μm. Lower: The mean number of Bax-positive cells in each group. (A) Control group, (B) control with postnatal exercise group, (C) maternal streptozotocin (STZ)-injection group, and (D) maternal STZ-injection with postnatal exercise group. Values shown are the mean± SEM. *P< 0.05 when compared to the control group. #P< 0.05 when compared to the maternal STZ-injection group. 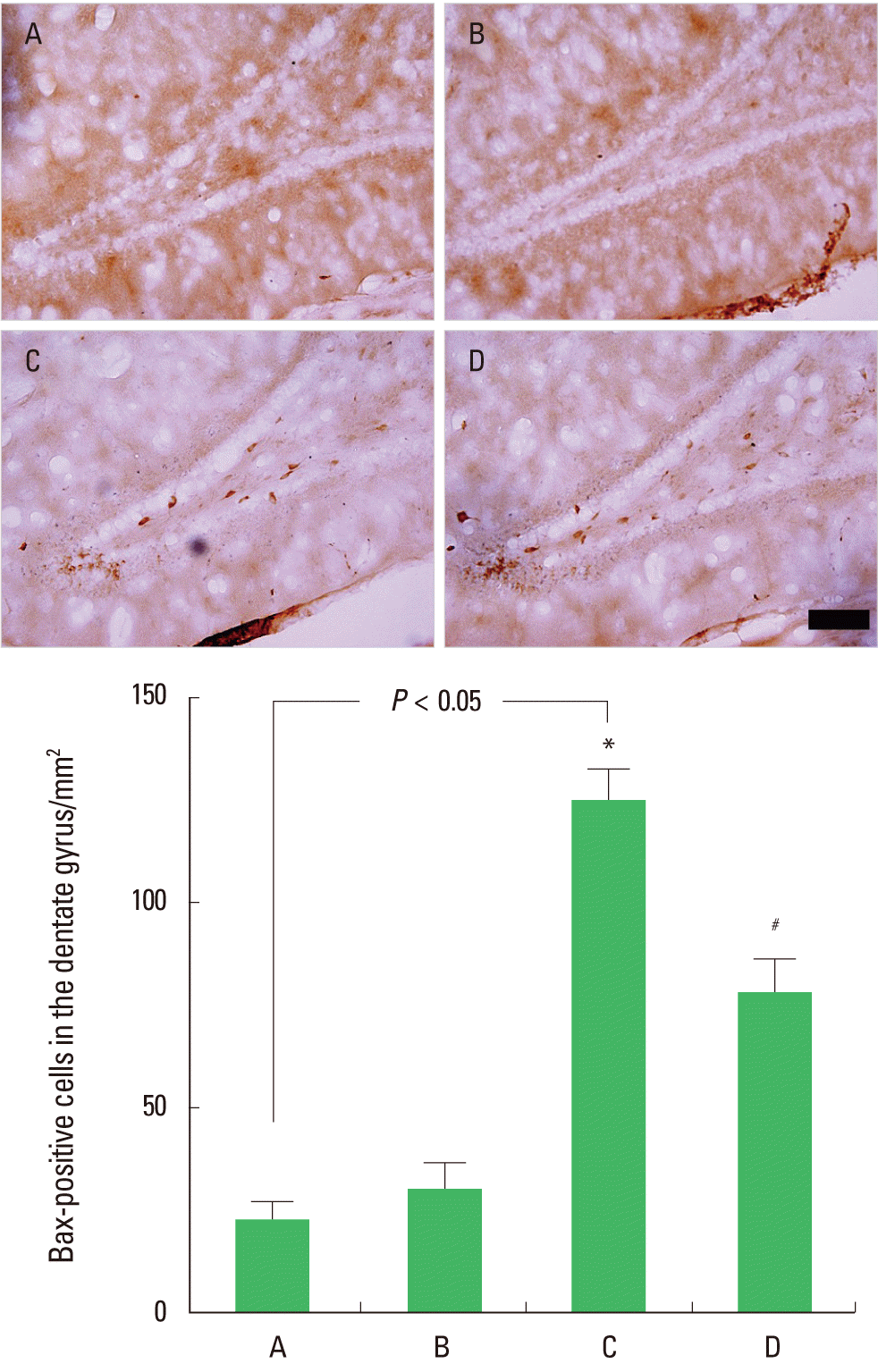
Fig. 5.Effect of postnatal treadmill exercise on DNA fragmentation in the hippocampal dentate gyrus. Upper: Photomicrograph showing immunostaining for terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) in dentate gyrus of the hippocampus. The scale bar represents 100 μm. Lower: The number of TUNEL-positive cells in each group. (A) Control group, (B) control with postnatal exercise group, (C) maternal streptozotocin (STZ)-injection group, and (D) maternal STZ-injection with postnatal exercise group. Values shown are the mean± SEM. *P< 0.05 when compared to the control group. #P< 0.05 when compared to the maternal STZ-injection group. 
|
|
|||||||||||||||||||||||||||||||||||||||