AbstractPerinatal hypoxic ischemia injury is a common cause of morbidity and mortality in neonates. Physical exercise may ameliorate neurological impairment by impeding neuronal loss following various brain insults. In the present study, the effect of treadmill exercise on sensory-motor function in relation with hippocampal apoptosis following hypoxic ischemia brain injury was investigated. Sensory-motor function was determined by walking initiation test and apoptosis was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining and caspase-3 immunohistochemistry. On postnatal 7 day, left common carotid artery of the neonatal rats was ligated for two hours and then the neonatal rats were exposed to hypoxia conditions for one hour. The rat pups in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 10 days, starting 22 days after induction of hypoxic ischemia brain injury. Hypoxic ischemia caused sensory-motor disturbance with enhancement of apoptosis in the hippocampus. Short-term treadmill exercise suppressed hypoxic ischemia injury-induced apoptosis in the hippocampus, and preserved sensory-motor function of hypoxic ischemia injury rat pups.
INTRODUCTIONPerinatal hypoxic ischemia injury is a serious problem and a common cause of morbidity and mortality in neonates. (Lubics et al., 2005). Perinatal exposure to hypoxic ischemia brain injury induces long-term neurological deficits (Jansen and Low, 1996), developmental disability (Dammann and Leviton, 1997), epilepsy and mental retardation (Papazisis et al., 2007), and long-term impact on intellectual function in children (Rennie et al., 2007).
Several interventions for the hypoxic ischemia have been suggested such as erythropoietin (Kim et al., 2008), fluoxetine (Chang and Huang, 2006), and hypothemia (Covey and Oorschot, 2007). The underlying mechanisms for the inhibition on hypoxic ischemia brain injury focused on the suppressing apoptosis (Parikh et al., 2003) and antagonizing N-methyl-D-aspartate (NMDA) receptor (Garcia de Arriba et al., 2006). Apoptosis is known as the forms of programmed cell death, and inhibition on apoptosis has been repeatedly proposed as a novel and promising therapeutic direction for neuronal rescue following neonatal hypoxic ischemia injury or stroke (Northington et al., 2005).
The hippocampus is noted as the particularly susceptible brain area to hypoxic, ischemic, and asphyctic brain injury (Scheepens et al., 2003). Hippocampus plays important roles in memory and spatial navigation. It has been suggested that physical exercise may ameliorate neurological impairment by impeding neuronal loss following various brain insults (Kim et al., 2007; Lee et al., 2005). Treadmill exercise inhibits hippocampal apoptosis (Yoon et al., 2007), and improves short-term memory (Sim et al., 2005).
However, the effect of treadmill exercise on sensory-motor function in relation with hippocampal apoptosis following hypoxic ischemia brain injury is currently unknown. For this, sensory-motor function was determined by walking initiation test and apoptosis was detected by terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining and caspase-3 immunohistochemistry using rats.
MATERNAL AND METHODSAnimals and treatmentsThe experimental procedures were performed in accordance with the animal care guidelines of National Institutes of Health (NIH) and Korean Academy of Medical Sciences. Rat pups were housed under the controlled temperature (20±2°C) and maintained in light-dark cycles, each cycle consisting of 12 h of light and 12 h of darkness (lights on from 07:00 h to 19:00 h). Food and water were made available ad libitum. Seven-day-old Sprague-Dawley rats, of both sex, weighing 10–15 g, were used for the experiments. This age is approximately equivalent to the 34-weeks old in human infants. The animals were divided into the four groups (n=10 in each group): the sham-operation group, the sham-operation and exercise group, the hypoxic ischemia-induction group, and the hypoxic ischemia-induction and exercise group.
Induction of hypoxic ischemia brain injuryFor the induction of hypoxic ischemia brain injury, a well-established model of perinatal brain damage was used (Rice et al., 1981). In this experimental procedure, brain injury is largely restricted to the cerebral hemisphere ipsilateral to the left common carotid artery occlusion (Vannucci et al., 1999).
On postnatal day 7, ischemia was induced by ligation of left common carotid artery. The neonatal rats were anaesthetized with diethyl ether and the midline of the neck was incised at the longitudinal plane. The left common carotid artery was exposed, dissected from the vagus nerve and doubly ligated with 4-0 silk suture to ensure cessation of blood flow. Total time of surgery was < 8 min. The rats were then allowed to recover with their dams, and two hours after surgery they were exposed to a hypoxia condition (8% oxygen, 92% nitrogen) for one hour by placing them in an airtight plastic box which was provided a 7°C stable thermal environment.
For the neonatal rats in the sham-operation group and in the sham-operation and exercise group, surgical incision was conducted without ligation and exposure to hypoxic condition was deducted. After exposure to hypoxia condition, the all neonatal rats were left undisturbed together with their respective mothers for 21 days.
Treadmill exercise protocolThe rat pups in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 10 consecutive days, starting 22 days after induction of hypoxic ischemia brain injury. The exercise load consisted of running at 2 meters/min, at 0 degree of inclination. This exercise regimen is a light-intensity exercise (Kim et al., 2004). The rat pups in the non-exercise groups were left in treadmill without running for the same period as the exercise groups.
Walking initiation testThis test assesses sensory-motor function after brain injury in rats (Lubics et al., 2005). The rats were placed on a horizontal surface in the center of two concentric circles with diameters of 20 cm and 60 cm. The time taken to move off the circles was recorded.
Tissue preparationThe rats were sacrificed after last exercise. At the beginning of the sacrificial procedure, the animals were weighed and overdosed with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France). After a complete lack of response was observed, the rats were transcardially perfused with 50 mM phosphate-buffered saline (PBS), and then with 4% paraformaldehyde in 100 mM phosphate buffer (PB) at pH 7.4. The brains were dissected, postfixed in the same fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40 μm thickness were made using a freezing microtome (Leica, Nussloch, Germany).
TUNEL stainingFor visualization of DNA fragmentation, a marker of apoptotic cell death, TUNEL staining was performed using In Situ Cell Death Detection Kit® (Roche, Mannheim, Germany) according to the manufacturer’s protocol (Ji et al., 2013; Sim et al., 2005). To begin the procedure, the sections were post-fixed in ethanolace tic acid (2:1) and rinsed. Then the sections were incubated with proteinase K (100 μg/mL), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in TUNEL reaction mixture. The sections were rinsed and visualized using Converter-POD with 0.02% 3,3′-diaminobenzidine (DAB). Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used for counter-staining, and the sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted using Permount®.
Caspase-3 immunohistochemistryFor visualization of the caspase-3 expression, caspase-3 immunohistochemistry was performed as previously described (Cho et al., 2013; Ji et al., 2013). The brain sections were incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then for another 1 h with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with Vector Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and the coverslips were mounted using Permount®.
Data analysesThe area of the granular layer of the dentate gyrus was measured using Image-Pro®Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA). The numbers of TUNEL-positive and caspase-3-positive cells were counted hemilaterally in each section and expressed as number of cells per mm2 of the cross-sectional area of the granular layer of the dentate gyrus. All data were analyzed using the statistical software SPSS (version 12.0). The data are expressed as the mean±standard error of the mean (SEM). For the comparison among the groups, one-way ANOVA and Duncan’s post-hoc test were performed and differences were considered statistically significant at P<0.05.
RESULTSEffect of short-term treadmill exercise on sensorimotor function in the 20 cm and 60 cm walking initiation testThe latency time of 20 cm walking initiation test was 1.29±0.47 sec in the sham-operation group, 0.54±0.07 sec in the sham-operation and exercise group, 2.81±0.62 sec in the hypoxic ischemia-induction group, and 1.53±0.25 sec in the hypoxic ischemia-induction and exercise group (Fig. 1, left panel). The latency time of 60 cm walking initiation test was 4.36±0.36 sec in the sham-operation group, 2.27±0.69 sec in the sham-operation and exercise group, 9.71±2.48 sec in the hypoxic ischemia-induction group, and 4.57±0.90 sec in the hypoxic ischemia-induction and exercise group (Fig.1, right panel). The time taken to move off a circle of 20 and 60 cm in diameter was decreased by induction of hypoxic ischemia brain injury, on the other hand, the time was increased by treadmill exercise (Fig. 1).
Effect of short-term treadmill exercise on the DNA fragmentation in the hippocampal dentate gyrusThe number of TUNEL-positive cells in the hipppocampal dentate gyrus was 4.31±1.20/mm2 in the sham-operation group, 11.98±1.88/mm2 in the sham-operation and exercise group, 41.11 ±5.83/mm2 in the hypoxic ischemia-induction group, and 24.25 ±2.30/mm2 in the hypoxic ischemia-induction and exercise group. Induction of hypoxic ischemia brain injury increased DNA fragmentation in the hippocampal dentate gyrus, on the other hand, DNA fragmentation was reduced by treadmill exercise (Fig. 2).
Effect of short-term treadmill exercise on the caspase-3 expression in the hippocampal dentate gyrusThe number of caspase-3-positive cells in the hippocampal dentate gyrus was 21.46±6.60/mm2 in the sham-operation group, 47.85±8.36/mm2 in the sham-operation and exercise group, 306.55±33.65/mm2 in the hypoxic ischemia-induction group, and 211.04±25.35/mm2 in the hypoxic ischemia-induction and exercise group. Induction of hypoxic ischemia brain injury increased caspase-3 expression in the hippocampal dentate gyrus, on the other hand, caspase-3 expression was reduced by treadmill exercise (Fig. 3).
DISCUSSIONHypoxic ischemia has been comprehensively studied since the 1950s and sporadically before this time (Golan and Huleihel, 2006). However the mechanisms responsible for the extensive neurodegeneration which occurs after hypoxia ischemia brain injury during brain development are not completely understood (Carloni et al., 2007). It is known that brain injury during the neonatal stage is caused, at least partly, by apoptosis (Gill, 2002; Nakajima et al., 2000; Zhao et al., 2007).
We focused on the anti-apoptotic effect of exercise. The present study demonstrated that induction of hypoxic ischemia enhanced DNA fragmentation and caspase-3 expression in the hippocampus, demonstrating that hypoxic ischemia injury-induced apoptotic neuronal cell death. Meanwhile short-term treadmill exercise suppressed hypoxic ischemia-induced DNA fragmentation and inhibited caspase-3 expression in the hippocampus, representing that short-term treadmill exercise showed inhibiting effect on hypoxic ischemia injury induced apoptotic neuronal cell death. TUNEL-positive cells represent apoptotic cell death and caspase-3 is one of the key executors of apoptosis (Cho et al., 2013; Ji et al., 2013). Consistent with other studies (Lee et al., 2005; Sim et al., 2005), short-term treadmill exercise also ameliorated apoptotic neuronal cell death caused by hypoxic ischemia brain injury.
In the present study, sensory-motor function in the walking initiation test was deteriorated by induction of hypoxic ischemia. Meanwhile short-term treadmill exercise alleviated hypoxic ischemia-induced sensory-motor impairment. Numerous studies dealing with severe perinatal hypoxic ischemia insults have out-lined the correlation between injury-associated cell loss and functional impairments (Ten et al., 2003; Derrick et al., 2004). In previous studies, hypoxic ischemia injury displayed short-term and long-term learning deficits in rodents (Lubics et al., 2005; Ten et al., 2003; Wagner et al., 2002; Wang et al., 2002). Exercise has been demonstrated to enhance cognition, neuronal plasticity, activity, and memory function (Kim et al., 2007; Sim et al., 2008; Stranahan et al., 2006; Vaynmanand, 2004).
The present study showed that hypoxic ischemia brain injury accelerated apoptotic neuronal cell death in the hippocampus and resulted in the sensory-motor dysfunction. Suppressing effect of short-term treadmill exercise on apoptosis ameliorated hypoxic ischemia-induced sensory-motor dysfunction. The present study suggests the possibility that short-term treadmill exercise in early period following neonatal hypoxic ischemia brain injury may provides a useful strategy for the recovery from the brain complications caused by hypoxic ischemia brain injury.
REFERENCESCarloni S, Carnevali A, Cimino M, Balduini W. Extended role of necrotic cell death after hypoxia-ischemia-induced neurodegeneration in the neonatal rat. Neurobiol Dis. 2007;27:354–361.
Chang YC, Huang CC. Perinatal brain injury and regulation of transcription. Curr Opin Neurol. 2006;19:141–147.
Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, Kim CJ. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopa-mine-induced Parkinson’s rats. J Exer Rehabil. 2013;9:354–361.
Covey MV, Oorschot DE. Effect of hypothermic post-treatment on hypoxic-ischemic striatal injury, and normal striatal development, in neonatal rats: a stereological study. Pediatr Res. 2007;62:646–651.
Dammann O, Leviton A. The role of perinatal brain damage in developmental disabilities, an epidemiologic perspective. Ment Retard Dev Disabil Res Rev. 1997;3:13–21.
Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34.
Garcia de Arriba S, Wegner F, Grüner K, Verdaguer E, Pallas M, Camins A, Wagner A, Wohlfahrt K, Allgaier C. Different capacities of various NMDA receptor antagonists to prevent ischemia-induced neurodegeneration in human cultured NT2 neurons. Neurochem Int. 2006;49:466–474.
Gill R, Soriano M, Blomgren K, Hagberg H, Wybrecht R, Miss MT, Hoefer S, Adam G, Niederhauser O, Kemp JA, Loetscher H. Role of caspase-3 activation in cerebral ischemia-induced neurodegeneration in adult and neonatal brain. J Cereb Blood Flow Metab. 2002;22:420–430.
Golan H, Huleihel M. The effect of prenatal hypoxia on brain development: short- and long-term consequences demonstrated in rodent models. Dev Sci. 2006;9:338–349.
Jansen EM, Low WC. Long-term effects of neonatal ischemic hypoxic brain injury on sensorimotor and locomotor tasks in rats. Behav Brain Res. 1996;78:189–194.
Ji ES, Ko IG, Cho JW, Davis RW, Hwang GY, Jee YS, Lim BV. Treadmill exercise inhibits apoptotic neuronal cell death with suppressed vascular endothelial growth factor expression in the retinas of the diabetic rats. J Exerc Rehabil. 2013;9:348–353.
Kim H, Lee SH, Kim SS, Yoo JH, Kim CJ. The influence of maternal tread-mill running during pregnancy on short-term memory and hippocampal cell survival in rat pups. Int J Dev Neurosci. 2007;25:243–249.
Kim SS, Lee KH, Sung DK, Shin JW, Kim MJ, Jeon GW, Chnag YS, Park WS. Erythropoietin attenuates brain injury, subventricular zone expansion, and sensorimotor deficits in hypoxic-ischemic neonatal rats. J Korean Med Sci. 2008;23:484–491.
Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, Lee SJ, Lee HH, Yoon JH, Jeong IG, Kim CJ. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett. 2004;355:152–154.
Lee HH, Shin MS, Kim YS, Yang HY, Chang HK, Lee TH, Kim CJ, Cho S, Hong SP. Early treadmill exercise decreases intrastriatal hemorrhage-induced neuronal cell death and increases cell proliferation in the dentate gyrus of streptozotocin-induced hyperglycemic rats. J Diabetes Complications. 2005;19:339–346.
Lubics A, Reglodi D, Tamás A, Kiss P, Szalai M, Szalontay L, Lengvári I. Neurological reflexes and early motor behavior in rats subjected to neonatal hypoxic-ischemic injury. Behav Brain Res. 2005;157:157–165.
Nakajima W, Ishida A, Lange MS, Gabrielson KL, Wilson MA, Martin LJ, Blue ME, Johnston MV. Apoptosis has a prolonged role in the neurodegeneration after hypoxic ischemia in the newborn rat. J Neurosci. 2000;20:7994–8004.
Northington FJ, Graham EM, Martin LJ. Apoptosis in perinatal hypoxic-ischemic brain injury: How important is it and should it be inhibited? Brain Res Brain Res Rev. 2005;50:244–257.
Papazisis G, Kallaras K, Kaiki-Astara A, Pourzitaki C, Tzachanis D, Dagklis T, Kouvelas D. Neuroprotection by lamotrigine in a rat model of neonatal hypoxic-ischaemic encephalopathy. Int J Neuropsychopharmacol. 2007;26:1–9.
Parikh NA, Katsetos CD, Ashraf QM, Haider SH, Legido A, Delivoria-Papadopoulos M, Mishra OP. Hypoxia-induced caspase-3 activation and DNA fragmentation in cortical neurons of newborn piglets: role of nitric oxide. Neurochem Res. 2003;28:1351–1357.
Rennie JM, Hagmann CF, Robertson NJ. Outcome after intrapartum hypoxic ischaemia at term. Semin Fetal Neonatal Med. 2007;12:398–407.
Rice JE 3rd, Vannucci RC, Brierley JB. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann Neurol. 1981;9:131–141.
Scheepens A, Wassink G, Piersma MJ, Van de Berg WD, Blanco CE. A delayed increase in hippocampal proliferation following global asphyxia in the neonatal rat. Brain Res Dev Brain Res. 2003;142:67–76.
Sim YJ, Kim H, Kim JY, Yoon SJ, Kim SS, Chang HK, Lee TH, Lee HH, Shin MC, Shin MS, Kim CJ. Long-term treadmill exercise overcomes ischemia-induced apoptotic neuronal cell death in gerbils. Physiol Behav. 2005;84:733–738.
Sim YJ, Kim H, Shin MS, Chang HK, Shin MC, Ko IG, Kim KJ, Kim TS, Kim BK, Rhim YT, Kim S, Park HY, Yi JW, Lee SJ, Kim CJ. Effect of postnatal treadmill exercise on c-Fos expression in the hippocampus of rat pups born from the alcohol-intoxicated mothers. Brain Dev. 2008;30:118–125.
Stranahan AM, Khalil D, Gould E. Social isolation delays the positive effects of running on adult neurogenesis. Nat Neurosci. 2006;9:526–533.
Ten VS, Bradley-Moore M, Gingrich JA, Stark RI, Pinsky DJ. Brain injury and neurofunctional deficit in neonatal mice with hypoxic-ischemic encephalopathy. Behav Brain Res. 2003;145:209–219.
Vannucci RC, Connor JR, Mauger DT, Palmer C, Smith MB, Towfighi J, Vannucci SJ. Rat model of perinatal hypoxic-ischemic brain damage. J Neurosci Res. 1999;55:158–163.
Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590.
Wagner BP, Nedelcu J, Martin E. Delayed postischemic hypothermia improves long-term behavioral outcome after cerebral hypoxia-ischemia in neonatal rats. Pediatr Res. 2002;51:354–360.
Wang LS, Zhou J, Shao XM, Tang XC. Huperzine A attenuates cognitive deficits and brain injury in neonatal rats after hypoxia-ischemia. Brain Res. 2002;949:162–170.
Fig. 1.Effect of short-term treadmill exercise on sensori-motor dunction. (Left) Latency in the 20 cm walking initiation test. (Right) Latency in the 60 cm walking initiation test. The data are presented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the sham-operation group. #P< 0.05 compared to the hypoxic ischemia-induction group. (A) Sham-operation group, (B) sham-operation and exercise group, (C) hypoxic ischemia-induction group, (D) hypoxic ischemia-induction and exercise group. 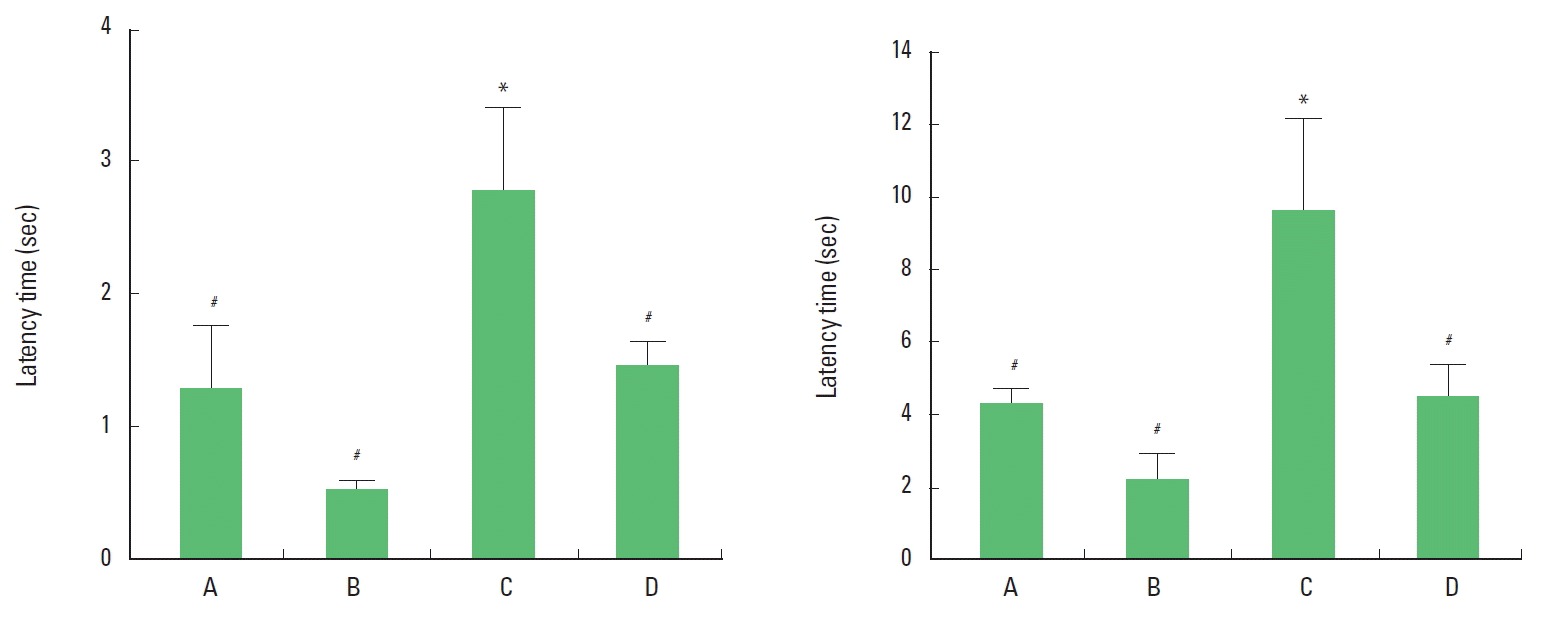
Fig. 2.Effect of short-term treadmill exercise on DNA fragmentation in the dentate gyrus. (Upper) Photomicrographs of terminal transferase-mediated dUTP nick end lebeling (TUNEL)-positive cells. The scale bar represents 25 μm. (Lower) Number of TUNEL-positive cells in each group. The data are presented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the sham-operation group. #P< 0.05 compared to the hypoxic ischemia-induction group. (A) Sham-operation group, (B) sham-operation and exercise group, (C) hypoxic ischemia-induction group, (D) hypoxic ischemia-induction and exercise group. 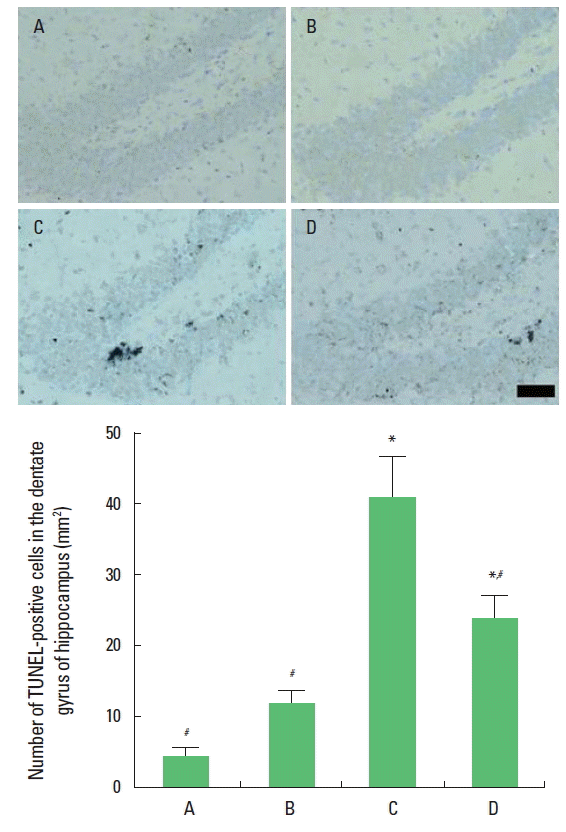
Fig. 3.Effect of short-term treadmill exercise on caspase-3 expression in the dentate gyrus. (Upper) Photomicrographs of caspase-3-postive cells. The scale bar represents 25 μm. (Lower) Number of caspase-3-postive cells in each group. The data are presented as the mean± standard error of the mean (SEM). *represents P< 0.05 compared to the sham-operation group. #P< 0.05 compared to the hypoxic ischemia-induction group. (A) Sham-operation group, (B) sham-operation and exercise group, (C) hypoxic ischemia-induction group, (D) hypoxic ischemia-induction and exercise group. 
|
|