INTRODUCTION
Post-traumatic stress disorder (PTSD) is a condition which occurs after a person has experienced unusual stress. People with PTSD become very anxious whenever reminded of the incident which has caused the initial distress. Frequently they have nightmares or become fearful, depressed and irritable and function less well at work or in social situations (Michael et al., 2006). Symptoms related to traumatic cues are expressed as conditioned or sensitized fear responses (Siegmund and Wotjak, 2006). The exaggerated fear memory, resulting from associative fear to traumatic cues and non-associative sensitization processes, is closely associated with PTSD (Wessa and Flor, 2007). The animal models of PTSD resemble the animal models of neurodegenerative disease (Hendriksen et al., 2010; Tamaki et al., 2008).
The hippocampus plays an important role in learning ability and memory capability (Milner et al., 1998). The neurons in the hippocampus are especially vulnerable to the PTSD (Hendriksen et al., 2010). Neurogenesis encompasses cell proliferation, survival, migration, and neuronal differentiation. Newborn neurons in the hippocampus is associated with the learning ability and memory function, and neurogenesis in the hippocampal dentate gyrus is known to be enhanced by many factors, such as enriched environment, neurotrophic factors, and exercise (Baek et al., 2012; Duman, 2005). The developmental stages of neurogenesis are characterized by stage-specific markers, including NeuroD, doublecortin (DCX), polysialylated neural cell adhesion molecule (PSANCAM), and calretinin (Ming and Song, 2005). Among these molecules, DCX, which is a marker of neuronal precursor cells, is associated with structural plasticity in the adult mammalian brain (Friocourt et al., 2007; von Bohlen und Halbach, 2011).
The beneficial effects of regular exercise on brain function and plasticity have been observed in many studies. Regular exercise attenuates motor deficits (Klintsova et al., 2002) and increases new neuron formation (Baek et al., 2012; van Praag et al., 2005). Physical exercise is currently advocated as a behavioral intervention to ameliorate neurological impairments by impeding neuronal loss following several neurodegenerative diseases (Duman, 2005; Kim et al., 2010). Availability of a running wheel, providing tunnels and shelters, and repeated introduction of novel objects lead to more opportunities to experience new sensory information, and then enhances neurogenesis in the hippocampal dentate gyrus of rats (Baek et al., 2012; van Praag et al., 2005).
Enhancing effect of exercise on neurogenesis has been well documented, however, the effect of treadmill exercise on PTSD-related neurogenesis has not been elucidated. In the present study, we investigated the effects of treadmill exercise on spatial learning memory and cell proliferation in the hippocampus of rats with PTSD. Radial 8-arm maze test and immunohistochemistr for 5-bromo-2′-deoxyridine (BrdU) and DCX were conducted for this experiment.
MATERIALS AND METHODS
Experimental animals and treatments
The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Male Sprague-Dawley rats, weighing 102.5±10 g (5 weeks old), were used in this experiment (Orient Co., Seoul, Korea). Each animal was housed under controlled temperature (20±2°C) and lighting (07:00–19:00 h) conditions with food and water made available ad libitum. The animals were randomly divided into 4 groups (n=10 in each group): the control group, the control and exercise group, the PTSD-induced group, and the PTSD-induced and exercise group.
Induction of PTSD
In order to induce PTSD in rats, the rats were exposure to the repeated inescapable electric foot shock, according to the previously described method (Li et al., 2005). The animal received 0.2 mA electric foot shock for 7 consecutive days. Electric foot shock continued 6 sec, repeated 10 times with a 30 sec interval per one trial, and repeated 3 trials per day.
Exercise protocol
The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 4 weeks, stating one day after finishing last electric food shock. The exercise load consisted of running at a speed of 5 meters/min for the first 5 min, 8 meters/min for the next 5 min, and 15 meters/min for the last 20 min, with a 0° inclination. The rats in the control group and in the PTSD-induced group were left on the treadmill without running for the same period as the exercise groups. BrdU (50 mg/kg; Sigma Chemical Co., St. Louis, MO, USA) was given intraperitoneally to all animals 1 h before the starting of treadmill running once a day for 3 consecutive days from the starting of treadmill exercise.
Radial 8-arm maze test
Spatial learning memory was evaluated using the radial 8-arm maze apparatus, as the previously described method (Kim et al., 2010). Radial 8-arm maze apparatus consisted of a central octagonal plate (30 cm in diameter) and 8 radiating arms (50 cm in length and 10 cm in width). The apparatus was placed 1 meter above the floor. A small receptacle filled with water (3 cm in diameter and 1 cm in depth) was located at the end of the arms. The rats were trained three times before the spatial learning memory test. The rats were deprived of water for 24 h and were allowed to explore the water for 5 min. Test was conducted 29 days after the starting of treadmill exercise. The time spent in seeking water at the end of the arms was counted. The test was terminated when a rat found water in all 8 arms or when >8 min elapsed. Re-entry into the previously visited arms was counted as an error. In addition, the number of correct choice before the first error was counted.
Tissue preparation
The animals were sacrificed immediately after finishing radial 8-arm maze test. The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phosphate buffer (PB, pH 7.4). The brains were dissected and postfixed in the same fixative overnight and transferred into a 30% sucrose solution for cryoprotection. Coronal sections of 40 μm thickness were made with a freezing microtome (Leica, Nussloch, Germany).
BrdU immunohistochemistry
BrdU-specific immunohistochemistry was performed, according to the previously described method (Baek et al., 2012). To begin the procedure, six sections on average were selected in each brain region spanning from Bregma −2.50 mm to −4.50 mm. The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min. The sections were then pretreated in 50% formamide-2 x standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Thereafter, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were washed three times with PBS and incubated for 1 h with a biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA). The sections were incubated with avidin-peroxidase complex (1:100; Vector Laboratories) for 1 h. For visualization, the sections were incubated in 50 mM Tris-HCl (pH 7.6) containing 0.02% 3,3′-diaminobenzidine tetrahydrochloride (DAB), 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide for 5 min.
After BrdU-specific staining, differentiation of BrdU-positive cells was determined on the same section using a mouse anti-neuronal nucleic (NeuN) antibody (1:300; Chemicon International, Temecula, CA, USA). The sections were washed three times with PBS and incubated for l h with a biotinylated anti-mouse secondary antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.02% DAB and 0.03% hydrogen peroxide for 5 min. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and coverslips were mounted with Permount®.
DCX immunohistochemistry
For visualization of the DCX expression, DCX immunohistochemistry was performed, as the previously described method (Friocourt et al., 2007). To begin the procedure, six sections on average were selected in each brain region spanning from Bregma −2.50 mm to −4.50 mm. The sections were incubated overnight with a mouse anti-DCX antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and then for another 1 h with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with a Vector Elite ABC kit (Vector Laboratories, Burlingame, CA, USA). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and the coverslips were mounted using Permount®.
Data analysis
The numbers of BrdU-positive and DCX-positive cells in the hippocampal dentate gyrus were counted using Image-Pro® Plus computer-assisted image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA) attached to a light microscope (Olympus, Tokyo, Japan). The numbers of BrdU-positive and DCX-positive cells were expressed as the numbers of cells per mm2 of cross-sectional area of hippocampal dentate gyrus. Statistical analysis was performed using the one-way ANOVA followed by Duncan’s post-hoc test, and the results are expressed as the mean± standard error of the mean (SEM). Significances were accepted to be present at P<0.05.
RESULTS
Effect of treadmill exercise on spatial learning memory in the radial 8-arm maze test
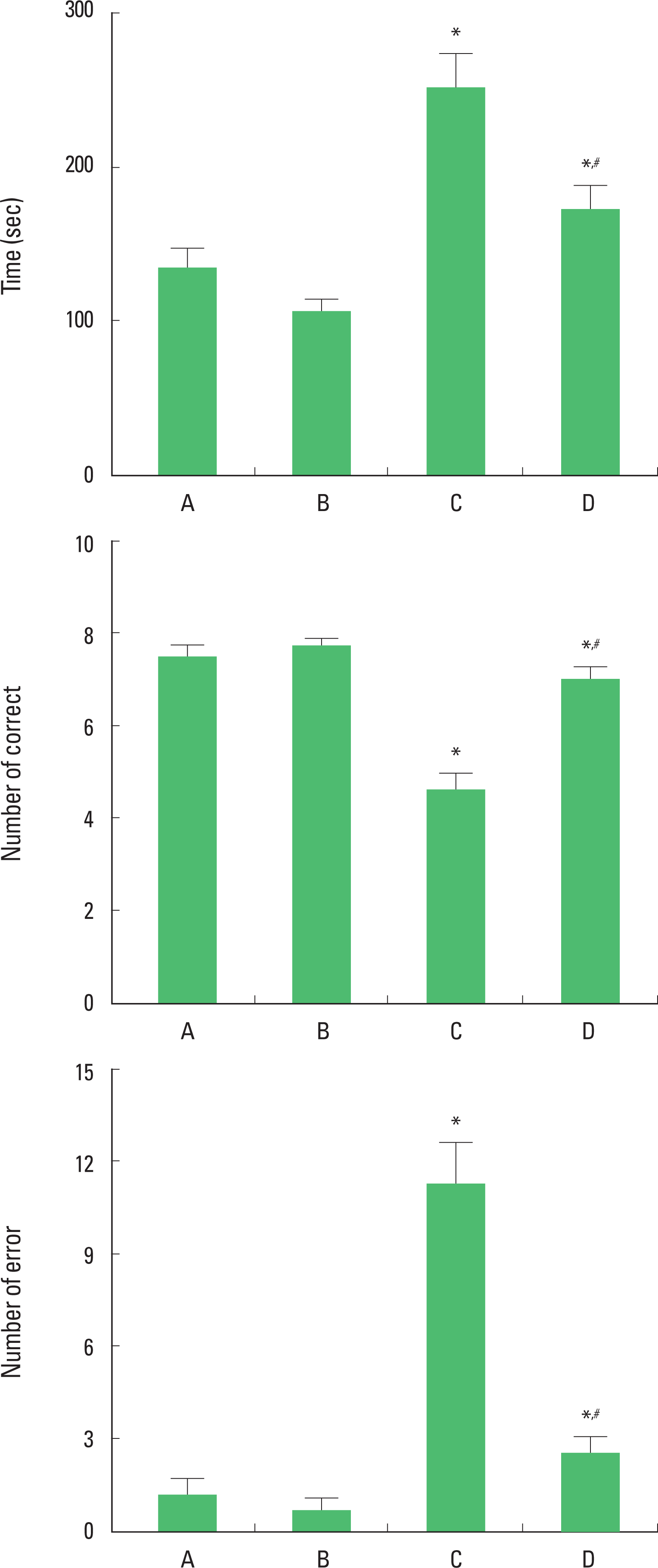
Spatial learning memory was evaluated using the radial-8-arm maze test. Time spent for completed eight successful performances was 134.90±11.25 sec in the control group, 106.70±6.26 sec in the control and exercise group, 250.60±22.56 sec in the PTSD-induced group, and 171.90±14.54 sec in the PTSD-induced and exercise group (Fig. 1, upper).
The number of correct choices before the first error was 7.50± 0.22 in the control group, 7.70±0.15 in the control and exercise group, 4.60±0.34 in the PTSD-induced group, and 7.00±0.21 in the PTSD-induced and exercise group (Fig. 1, middle).
The number of errors made before eight successful performances was 1.20±0.53 in the control group, 0.70±1.16 in the control and exercise group, 11.30±1.34 in the PTSD-induced group, and 2.50±0.54 in the PTSD-induced and exercise group (Fig. 1, lower).
The rats in the PTSD-induced group showed longer time for the successful performances, lower number of correct choices, and higher number of errors compared to the rats in the control group. However, treadmill exercise reduced time of the successful performance, increased correct number, and reduced error number in the rats with PTSD.
Effect of treadmill exercise on cell proliferation in the hippocampal dentate gyrus
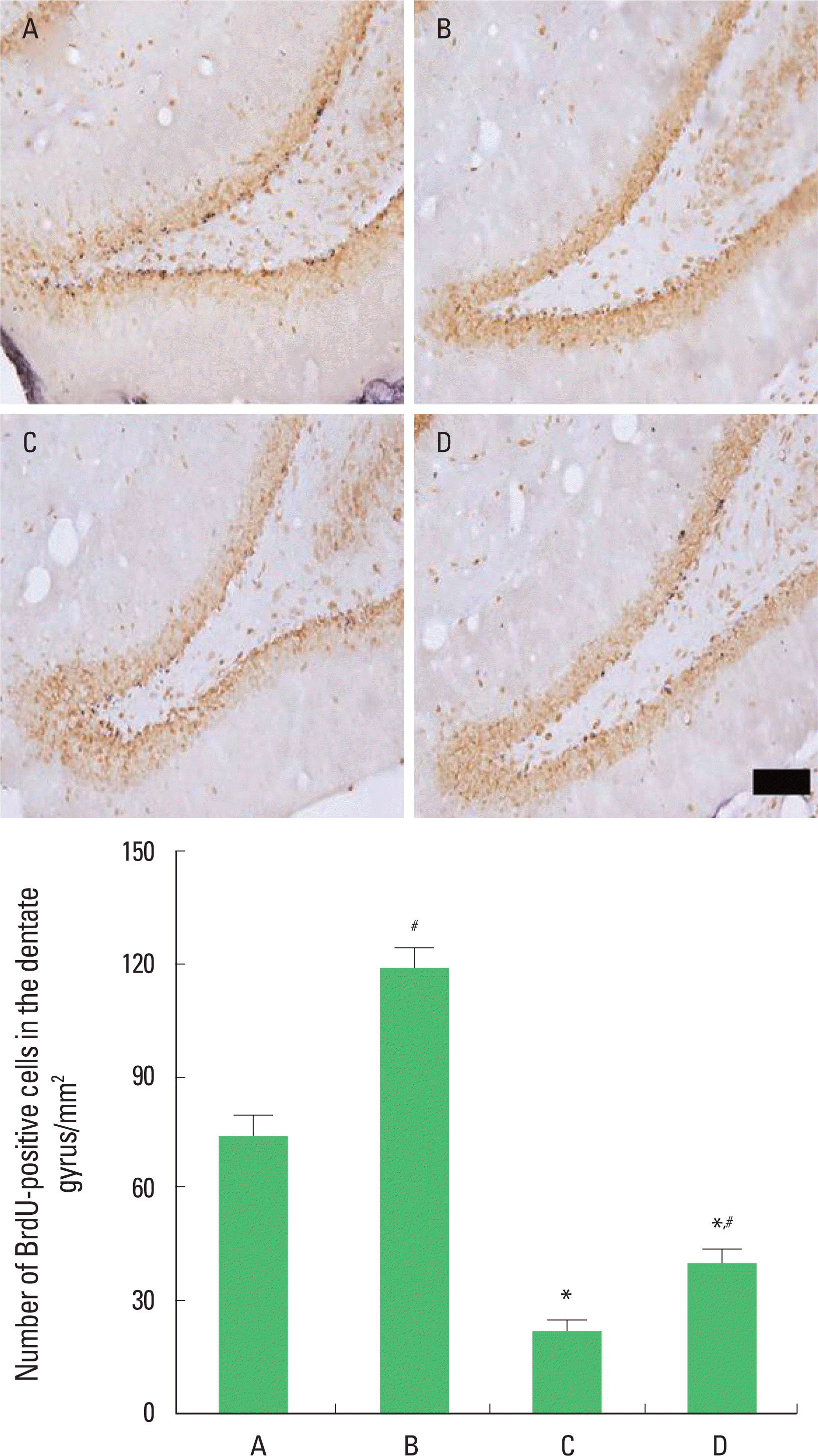
Photomicrographs of BrdU-positive cells in the hippocampal dentate gyrus are presented in Fig. 2. The number of BrdU-positive cells was 74.25±4.86/mm2 in the control group, 118.77± 4.93/mm2 in the control and exercise group, 22.10±2.40/mm2 in the PTSD-induced group, and 39.70±3.33/mm2 in the PTSD-induced and exercise group.
The present results show that inducing of PTSD decreased cell proliferation in the hippocampal dentate gyrus and that treadmill exercise increased cell proliferation in the rats with PTSD.
Effect of treadmill exercise on DCX expression in the hippocampal dentate gyrus
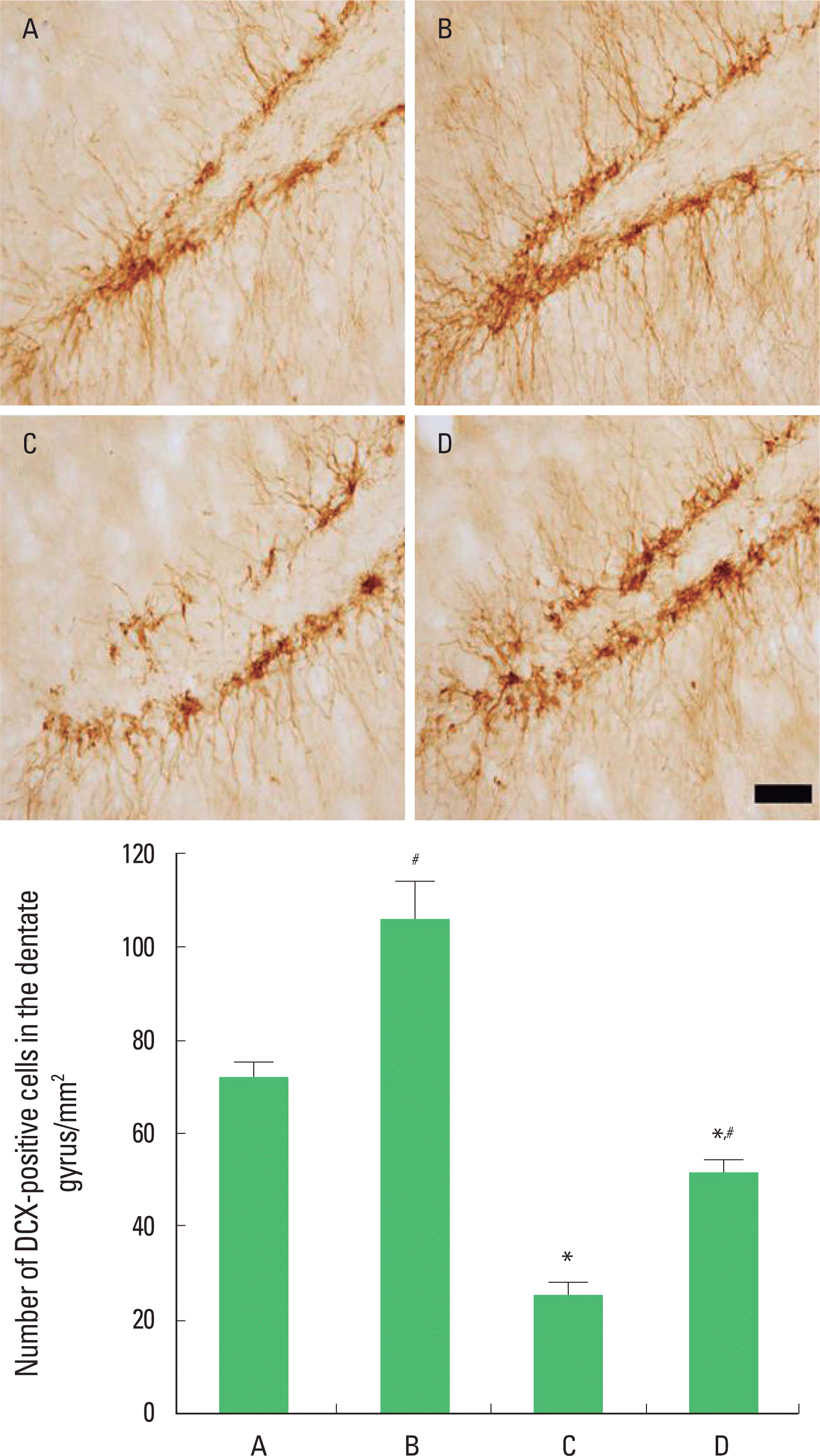
Photomicrographs of DCX-positive cells in the hippocampal dentate gyrus are presented in Fig. 3. The number of DCX-positive cells was 72.20±3.15/mm2 in the control group, 106.14±7.96/mm2 in the control and exercise group, 25.15±2.45/mm2 in the PTSD-induced group, and 51.27±2.62/mm2 in the PTSD-induced and exercise group.
The present results show that inducing of PTSD decreased DCX expression in the hippocampal dentate gyrus and that treadmill exercise significantly increased DCX expression in the rats with PTSD.
DISCUSSION
PTSD is induced by exposure to the severe traumatic events that occur during war, after violent personal assault, and following natural disasters. Inescapable electric foot shock also induces behavioral and biological changes similar to PTSD (Li et al. 2005; Milad et al. 2006). PTSD-induced animals exhibit severe degenerative changes and neuronal cell loss in sensory and autonomic nervous system (Hendriksen et al., 2010; Michael et al., 2006). It has been suggested that excessive stress is known to cause deteriorative effect on cognition and memory in humans and animal models (Stawski et al., 2006). Elizalde et al. (2008) reported that chronic mild stress also induces recognition memory deficits. Exposure to PTSD and chronic stress lead to dendrite atrophy and neuronal cell death associated with memory impairment and behavioral abnormalities (Lucassen et al., 2010). In the present study, the PTSD rats showed longer time of successful performance, higher error number, and lower correct number in the radial-8-arm maze test, which indicate deterioration of spatial learning memory.
Atrophy of the hippocampus was observed who experienced severe, long-lasting traumatic stress (Karl et al., 2006). Memory dysfunction in aged rats was quantitatively related to the hippocampal neurogenesis, suggesting that neurogenesis is involved in memory processes and aged-related cognitive alterations (Drapeau et al., 2003). Newly generated neurons in the adult brain are essential for the formation of new memories (Schmidt-Hieber et al., 2004). Neurogenesis is modulated by a variety of factors including stress, an enriched environment, learning, serotonin (Baek et al., 2012; Fuchs et al., 2001; Shors et al., 2011). Stress inhibits cell proliferation of new granular cells in the hippocampal dentate gyrus (Fuchs et al., 2001; Shors et al., 2011). Prolonged elevation of glucocorticoid hormones results in reduced hippocampal volume, fewer hippocampal neurons, and decreased neurogenesis (Hendriksen et al., 2010; Tamaki et al., 2008; Wignall et al., 2004). Chronic restraint stress decreased markers of synaptic plasticity in the hippocampus of rats (Rosenbrock et al., 2005). Randomly-scheduled foot shock followed by restraint in water, which was used as the stress-provoking regimen, rapidly decreased BrdU/DCx-labeled cells in the hippocampal dentate gyrus (Cherng et al., 2010). In the present study, cell proliferation and DCX expression in the hippocampal dentate gyrus was suppressed in the PTSD rats.
Exercise is known to ameliorate memory impairment and exerts protective effects against various neuropsychiatric diseases (Baek et al., 2012; Cho et al., 2013; Kim et al., 2010; van Praag et al., 2005). Recently, Seo et al. (2013) reported that treadmill exercise during pregnancy reduced PTSD-induced anxiety-like behaviors in maternal rats. In the present study, treadmill exercise alleviated PTSD-induced impairment of spatial learning memory, longer time of successful performance, higher error number, and lower correct number in the radial-8-arm maze test. These results suggest that treadmill exercise overcame the PTSD-induced deterioration of spatial learning memory.
Exercise is one of the best-known stimulators of adult hippocampal neurogenesis (Baek et al., 2012; Kim et al., 2010; van Praag et al., 2005). Voluntary wheel running reversed the decline in neurogenesis in aged mice to 50% of young control levels (van Praag et al., 2005). Treadmill exercise improved short-term and spatial memories by enhancing neurogenesis in aged rats (Kim et al., 2010). Postnatal treadmill exercise alleviated maternal separation-induced impairment of memory function through enhancement of cell proliferation in the hippocampus (Baek et al., 2012). DCX is a brain-specific microtubule-associated protein, and DCX is expressed during neurogenesis by mitotic and early postmitotic neurons (Friocourt et al., 2007; von Bohlen und Halbach O, 2011). DCX-immunoreactivity, a marker of progenitors differentiating into neurons, in the hippocampal dentate gyrus was markedly increased by treadmill exercise (Yi et al., 2009). Increased numbers of BrdU-positive and DCX-positive cells in the hippocampal dentate gyrus represent that exercise enhanced neuronal plasticity (Ferreira et al., 2011). In the present study, treadmill exercise enhanced cell proliferation and DCX expression in the hippocampal dentate gyrus of PTSD rats. These results suggest that treadmill exercise counteracted to the PTSD-induced suppression of neuronal plasticity.
The present study demonstrated that treadmill exercise ameliorated PTSD-induced memory impairment through enhancing cell proliferation in the hippocampus. Based on the present result, treadmill exercise may be useful strategy for the symptom relief on stress-induced neurpsychiatric disorders including PTSD.