INTRODUCTION
Spinal cord injury is a major medical problem worldwide, and realistic goals of functional repair have only recently been acknowledged. This study reviews the role of transplantation, focusing on stem cells and peripheral nerve transplantation and transfer. Neural stem/progenitor cells (NSPCs) have previously been identified in both the mammalian brain and spinal cord (Horner et al., 2000; Johansson et al., 1999; Namiki et al., 1999; Weiss et al., 1996). They have the ability to self-renew and are multipotential for both neurons and glia. Because of these qualities, they have been useful for repair of the spinal cord by generating new cells and an environment that would promote axonal regeneration (McDonald et al., 1999; Teng et al., 2002; Vroemen et al., 2003). However, this regenerative ability of endogenous stem/progenitor cells in mammals appears to be limited as proliferation and differentiation cease within a few days of trauma, providing only small numbers of new cells (Namiki et al., 1999). It has been reported that poor NSPC survival, even under optimized conditions for timing and location of transplantation, with large cavities and only a small number of surviving NSPCs located near healthier tissue (Parr et al., 2007). Therefore, a potential alternative source of NSCs is from blastocyst-derived cells, which are expanded as totipotent embryonic stem (ES) cells (Svendsen and Smith, 1999). Induction of ES cells into committed precursors can yield purified populations of NSCs, precursors, or differentiated neural cell types (Svendsen and Smith, 1999). The resulting stem cells can be expanded in culture as neurospheres which may then be used for transplantation. Grafting of neural differentiated mouse ES cells into a rat thoracic spinal cord clip-compression injury resulted in the survival, migration, differentiation into astrocytes, oligodendrocytes and neurons, and improved locomotor function (McDonald et al., 1999). The objectives in the present experiments were to examine functional recovery at 35 days after transplantation of spinal cord derived mESCs into the injured adult rat spinal cord (35 g injury) with and without prior transplantation. In the future experiment, cells from transgenic rats expressing the gene for enhanced green fluorescent protein (eGFP) would be used to identify the transplanted cells.
MATERIALS AND METHODS
Cell culture
ES cell cultures were prepared from stocks of an EK1 cell line (TC-1 derived from 129S6) maintained in our laboratory. Not more than 40 passages were used for experiments. The passage procedure of undifferentiated ES cells was performed every 2 days on gelatin-coated T25 flasks in the presence of 1,000 U/mL of leukemia inhibitory factor from Chemicon International (LIF) (LIF 2010, Temecula, CA, USA) and high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) (GibcoBRL, Germany) with 15% FBS (Hyclone), 0.1 mM mercaptoethanol, 1 μM sodium pyruvate, 1× non-essential amino acids and 1 mM L-glutamine (GibcoBRL). Briefly, ES cells were harvested from T25 flasks by trypsinization with 0.25% trypsin and placed into a standard 100-mm bacterial Petri dish in ESIM without adding LIF or β-mercaptoethanol. Medium was removed and cells were resuspended in modified Sato medium. Cells were then plated on poly-D-lysine (PDL) and laminin coated 35-mm glassbottom dishes for imaging studies or 24-well plates in preparation for serum deprivation (SD) experiments (Fig. 1).
Spinal cord injury model
Male Sprauge-Dawley rats were used for the experiments (7 weeks old at time of injury, 180–220 g, n=45). This study was approved by the animal care and use committee of Namseoul university. The transplant group (n=30) received both SCI and cell transplantation and the transplant control group (n=15) got SCI and PBS injection. The animals were maintained in a 12-h light/dark cycle with water and food freely available. The animals were fasted for 12 h before surgery, humanely restrained and anesthetized with an i.p. injection of pentobarbital sodium (50 mg/kg of body weight). Rectal temperature was maintained at 37–38°C by exposing the animal to a heat lamp throughout the operative procedure as needed until they completely recovered from anesthesia. The animals were positioned in the prone position and surgery was performed under sterile conditions. The clip compression injury was performed at the region of the 9–10th thoracic segment by removing the dorsal processes of the 9th and 10th thoracic vertebrae. The clip used to produce SCI was a modified vascular aneurysm clip, which has already been applied to produce SCI in rats (Euler et al., 1997; Joshi and Fehlings, 2002). The spinal cord was compressed for 1 min dorsoventrally. The animals received postoperative care including subcutaneous administration of gentamycin (5 mg/kg) daily for 5 days. Urinary bladders were emptied by abdominal compression at least twice daily.
Implantation procedure
The sham control group (n=15) was performed to determine if the injection volume (10 μL) or transplantation procedures triggered the observed behavioral changes in SCI rats. Sham control injection rats were subjected to SCI and injections of PBS (10 μL) after post-injury. However, there were no behavioral changes in SCI rats in relation to the injection procedures. Acute clip-compression injury of the thoracic spinal cord was conducted with a 35 g clip. After laminectomy, the exposed spinal cord was compressed at the T10 level by a 35 g clip. Cell transplantation was performed 7 days after injury. Rats of the transplant group received a total volume of 10 mL of cell suspension (1×105 cells/mL) in the lesioned cord. All animals were provided prophylactic antibiotics and a daily injection of cyclosporine A (10 mg/kg, s.c.), beginning 1 day before transplantation until the end of the experiment.
Behavioral assessment
Functional tests were performed before the injury and transplantation, and then daily for 35 days before and after transplantation. Locomotor activity was evaluated using the BBB locomotor rating scale for 4 min. Two independent blinded examiners observed and video recorded hind-limb movements and assessed the animal’s locomotor function (Basso et al., 1994). Motor subscores were also determined according to the method of (Lankhorst et al., 1999). Ladder-walk analysis was also recorded and analyzed weekly utilizing the apparatus described by (Metz and Whishaw, 2002). Rats were trained for 1 week prior to injury to traverse a horizontal ladder. Recordings were analyzed in slow motion and the number of footfalls for each hind limb was recorded and the average was calculated for each rat, each day.
Morphology
At eight weeks following SCI, all animals were transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer after being anesthetized. The spinal cord was dissected and postfixed for 24 h in the same fixative, then placed in 30% sucrose PBS solution for another 24 h. The portions of the spinal cord corresponding to the area of the injury site and transplant region were frozen. 10 μm thick serial longitudinal sections were obtained and stained with hematoxylin and eosin. The entire area of injury was visualized and stained to determine the true epicenter of the injury. The sections at every 30 μm in the rostral and caudal directions of the injury epicenter were examined under at 40×100× and 200×magnification using bright field and fluorescence microscopy with stained H–E for the visual analyses of cavity size.
RESULTS
Formation of cavity volume
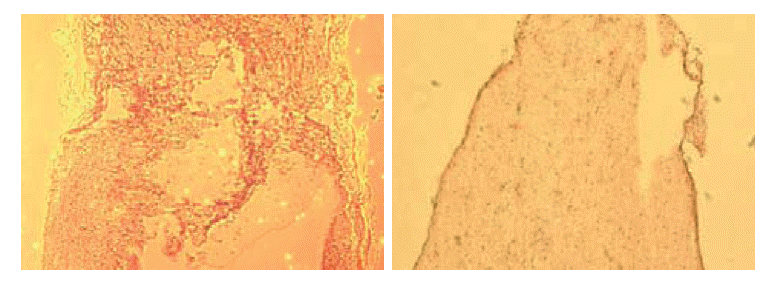
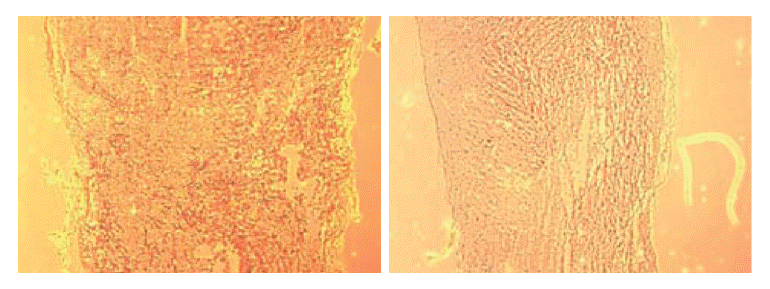
Non-transplant animals with BBB scores less than 7 showed the formation of large cavities (Fig. 2). The spinal cords of mESC-transplant animals had cavities much smaller than those of non-transplant animals (Fig. 3). These results suggested that mESC-transplant reduced the formation of cavities after injury in the SCI model.
Functional analysis
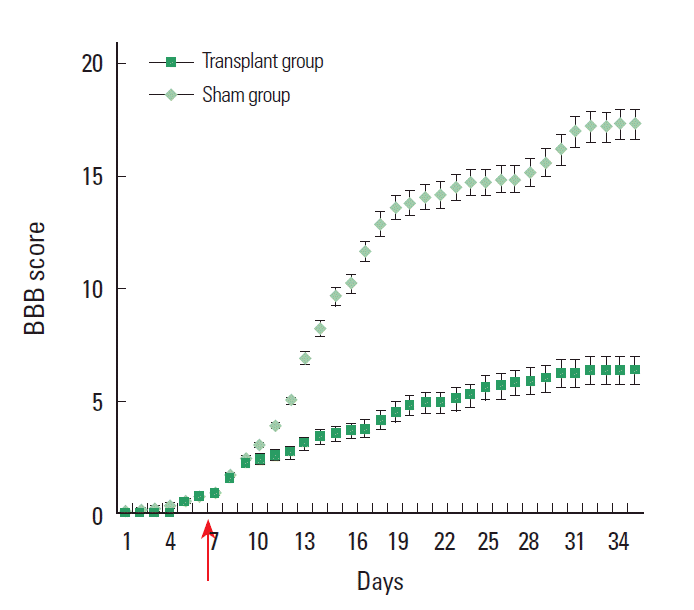
Locomotor performance evaluated daily by BBB scoring showed a significant improvement in rats receiving mESCs only (Fig. 4) compared with control group. This improvement, compared with the medium only control group, reached significance at 2 weeks, although an early trend was noted. Locomotor performance was also evaluated daily by motor subscoring. After the transplant of mESC, transplant animals showed a significant functional improvement of BBB scores as compared to non-transplant animals at all time point examined.
DISCUSSION
Transplantation of stem cells and nerve grafts and transfer of peripheral nerves represent important new approaches to managing spinal cord injury. Improvements in molecular and microscopic techniques along with the availability of modified stem cell lines have accelerated research into neural stem cell transplantation. We found that transplanted mESCs produced significant functional improvement after clip-compressive SCI. Indeed, all functional tests showed significant improvement in rats receiving mESCs compared with control group. It is of interest that most studies reporting functional improvement after cell transplantation showed early onset of improvement within the first 2–3 weeks after injury which suggests a neuroprotective rather than a regenerative mechanism (Chopp et al., 2000; Teng et al., 2002; Vacanti et al., 2001). However, it is also notable that many transplanted rats had very few transplanted cells surviving after several weeks, suggesting that the cells do not need to survive long term to produce functional recovery (Hofstetter et al., 2002, 2005).
Thus, transplantation of mESCs and earlier cell environment produced a major improvement in function after SCI. It is most likely that these salutary effects are manifestations of neuroprotection, although it is unknown whether this is due to growth factors or other agents elaborated by the transplanted cells. Also, although we may suggest an important role in the early effect of mESCs, this does not rule out a regenerative component to account for further functional recovery. Thus regeneration remains a possibility to be examined in future studies with transplanted spinal cord-derived mESCs. In conclusion, we may suggest that various embryonic stem cells transplantation therapy could be a potential benefit for clinical application in spinal cord injury disease.