AbstractProgressive loss of dopaminergic neurons in substantia nigra is a key pathogenesis of Parkinson’s disease. In the present study, we investigated the effects of treadmill exercise on short-term memory, apoptotic dopaminergic neuronal cell death and fiber loss in the nigrostriatum, and cell proliferation in the hippocampal dentate gyrus of Parkinson’s rats. Parkinson’s rats were made by injection of 6-hydroxydopamine (6-OHDA) into the striatum using stereotaxic instrument. Four weeks after 6-OHDA injection, the rats in the 6-OHDA-injection group exhibited significant rotational asymmetry following apomorphine challenge. The rats in the exercise groups were put on the treadmill to run for 30 min once a day for 14 consecutive days starting 4 weeks after 6-OHDA injection. In the present results, extensive degeneration of the dopaminergic neurons in the substantia nigra with loss of dopaminergic fibers in the striatum were produced in the rats without treadmill running, which resulted in short-term memory impairment. However, the rats performing treadmill running for 2 weeks alleviated nigrostriatal dopaminergic cell loss and alleviated short-term memory impairment with increasing cell proliferation in the hippocampal dentate gyrus of Parkinson’s rats. The present results show that treadmill exercise may provide therapeutic value for the Parkinson’s disease.
INTRODUCTIONParkinson’s disease is induced by degeneration of neurons in the substantia nigra. Clinical manifestations of Parkinson’s disease are tremor at rest, muscle rigidity, postural instability, an inability to initiate or complete movements, and a lack of facial expression as well as cognitive and vegetative disturbances (Fahn, 2003). Parkinson’s disease patients show impairment in working and episodic memory and procedural learning ability (Dujardin and Laurent et al., 2003). Memory impairment is associated with hippocampal atrophy in Parkinson’s disease (Riekkinen et al., 1998; Camicioli et al., 2003). The hippocampus plays an important role in learning and memory.
Apoptosis, known as programmed cell death, is enhanced by a variety of pathologic stimuli. He et al. (2000) showed that intra-cerebral injection of 6-hydroxydopamine (6-OHDA) caused apoptotic cell death of dopaminergic neurons in the substantia nigra of rats. Apoptotic cell death is implicated in the pathogenesis of Parkinson’s disease (Lev et al., 2003). Caspases, which make up a family of cysteinyl proteases encompassing 14 members, are essential players in apoptotic cell death both as initiators (caspase-2, -8, -9, and -10) and executioners (caspase-3, -6, and -7). It has also been reported that caspases-3 is activated following Parkinson’s disease (Hartmann et al., 2000).
Cell proliferation and/or neurogenesis in the hippocampal dentate gyrus continue throughout life in the adult mammals including humans (Eriksson et al., 1998). New cell formation in the hippocampal dentate gyrus is increased by learning, memory, serotonin, N-methyl-D-aspartate (NMDA) receptor antagonists, and exposure to an enriched environment including physical exercise (Fuchs and Gould, 2000). van Praag et al. (1999) reported that voluntary wheel running increased cell proliferation and survival in mice, and Trejo et al. (2001) showed that treadmill exercise enhanced cell proliferation in the hippocampal dentate gyrus in rats.
Physical exercise improved motor performance, enhanced cognitive function, and reduced mortality and incidence of Parkinson’s disease (Chen et al., 2005; Miyai et al., 2000). Exercise facilitated functional recovery after striatal lesions and striatal grafts (Döbrössy and Dunnett, 2003), and exercise stimulated dopamine synthesis in the striatum of epileptic mice (Sutoo and Akiyama, 2003). Rehabilitative motor training attenuated the behavioral and neuro-chemical deficits induced by 6-OHDA injection in rats (Cohen et al., 2003; Tillerson et al., 2003). Special movement therapy improved short-term memory in Parkinson’s disease patients (Schalow et al., 2005).
Endogenous 6-OHDA is a neurotoxin which selectively injures catecholaminergic neurons (Jellinger et al., 1995) and it causes progressive loss of nigral dopaminergic neurons (Blum et al., 2001). The rats receiving intrastriatal infusion of 6-OHDA have widely been used as the animal model of Parkinson’s disease (Yoon et al., 2007). Tyrosine hydroxylase (TH) is the rate-limiting enzyme in the synthesis of the catecholamine neurotransmitters such as dopamine, epinephrine, and norepinephrine. TH activity is progressively decreased following the loss of dopamine neurons in the substantia nigra of the Parkinson’s disease (Yoon et al., 2007).
In the present study, we investigated whether treadmill exercise exerts therapeutic effects on symptoms of Parkinson’s disease after onset of this disease. The effects of treadmill exercise on short-term memory, apoptotic dopaminergic neuronal cell death and fiber loss in the nigrostriatum, and cell proliferation in the hippocampal dentate gyrus of 6-OHDA-induced Parkinson’s rats were evaluated.
MATERIALS AND METHODSAnimals and treatmentsAdult female Sprague-Dawley rats weighing 200±10 g (6 weeks in age) were used in this experiment. The rats were housed at a room temperature (20±2°C) under standard 12 h light/dark cycles (lights on at 07:00 a.m.). Food and water were made available ad libitum. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The animals were randomly assigned into four groups (n=10 in each group): the sham-operation group, the sham-operation and exercise group, the 6-OHDA-injection group, and the 6-OHDA-injection and exercise group.
The rats in the 6-OHDA-injection groups were anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), and placed in a stereotaxic frame. Through a hole drilled in the skull, a 26-gauge needle was implanted into the striatum at the following coordinates: 3.0 mm lateral to midline, 0.0 mm anterior to coronal suture, depth 6.0 mm deep from the surface of the brain. And then, 6-OHDA (20 μg/5 μL) containing 0.2 mg/mL L-ascorbic acid was injected at the rate of 1 μL/min. The needle remained in place for an additional 5 min following the infusion, and then was slowly withdrawn. The animals in the sham operation groups were injected with an equivalent dose of physiological saline with the same method.
Apomorphine-induced rotation testFour weeks after unilateral 6-OHDA injection into the striatum, the changes of rotational behavior induced by apomorphine (0.5 mg/kg, s.c.) were assessed using an automatic rotometer over 60 min period as the previously described method (Yoon et al., 2007). The net number of rotations was counted as follows: the number of contralateral rotation - the number of ipsilateral rotation with respect to the 6-OHDA injection side.
Exercise protocolThe animals in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 14 consecutive days starting 4 weeks after 6-OHDA injection. The exercise load consisted of running at a speed of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 20 min. The animals in the non-exercise groups remained in the treadmill for the same duration of time without running. To all animals, 50 mg/kg 5-bromo-2′-deoxyuridine (BrdU, Sigma Chemical Co., St. Louis, MO, USA) was given intraperitoneally 1 h before the starting of treadmill running once a day for the same duration of treadmill exercise.
Step-down avoidance taskIn order to evaluate the short-term memory, we first determined the latency of the step-down avoidance task, according to the previously described method (Kim et al., 2013). On the 12th day from the beginning of the treadmill exercise, the rats were trained on a step-down avoidance task. The rats were placed on the 7×25 cm platform, 2.5 cm in height and allowed to rest on the platform for 2 min. The platform faced a 42×25 cm grid of parallel 0.1 cm-caliber stainless steel bars spaced 1 cm apart. In training sessions, the animals received 0.3 mA scramble foot shock for 2 sec immediately upon stepping down. Retention time was determined on the 14th day from the commencement of treadmill exercise. The interval of rats stepping down and placing all four paws on the grid was defined as the latency of step-down avoidance task. The latency over 300 sec was counted as 300 sec.
Tissue preparationAfter behavior test, the animals were deeply anesthetized with Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phosphate-buffered saline (PBS) and fixed with 4% paraformaldehyde (PFA) in 100 mM phosphate buffer (PB) at pH 7.4. The brain were removed, postfixed in the fixative overnight, and transferred into a 30% sucrose solution for cryoprotection. Serial coronal sections of 40 μm thick were made with a freezing microtome (Leica, Nussloch, Germany).
TH immunohistochemistryThe sections in the substantia nigra was selected from the region spanning from Bregma −5.2 mm to −5.6 mm, and TH-immunohistochemistry was performed as the previously described method (Kim et al., 2012; Yoon et al., 2007). The staining was carried out using free-floating sections. The sections were rinsed in PBS and incubated in 3% H2O2 for 20 min to block the endogenous peroxidase activity. After washing in PBS, the sections were incubated in blocking serum (10% normal horse serum and 0.1% Triton X-100 in PBS) for 30 min, followed by incubation in anti-TH mouse monoclonal antibody solution (1:1,000; Chemicon, Temcula, CA, USA) for 24 h at room temperature. The sections were then incubated for 1 h in biotinylated anti-mouse IgG secondary antibody (1:300; Vector Laboratories, Burlingame, CA, USA). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% 3,3-diaminobenzidine (DAB) and 0.01% H2O2 in 50 mM Tris buffer (pH 7.6) for 3 min. The sections were mounted on gelatine-coated slides and cover slipped with mounting medium.
For TH-immunohistochemistry in the striatum, the sections from Bregma 0.1 mm to −0.1 mm were selected to quantify the optical densities of TH-immunoreactive fibers, according to the previously described method (Kim et al., 2012; Yoon et al., 2007). TH-immunoreactive fiber density was measured in 100×100 μm square images of the striatum using an image analyzer (Multiscan, Fullerton, CA, USA).
Caspase-3 immunohistochemistryFor visualization of the caspase-3 expression in the substantia nigra, caspase-3 immunohistochemistry was performed as previously described method (Lee et al., 2003). The brain sections were incubated overnight with mouse anti-caspase-3 antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and then for another 1 h with the biotinylated mouse secondary antibody. The bound secondary antibody was then amplified with Vector Elite ABC kit® (Vector Laboratories). The antibody-biotin-avidin-peroxidase complexes were visualized using 0.02% DAB. The sections were finally mounted onto gelatin-coated slides. The slides were air dried overnight at room temperature, and the coverslips were mounted using Permount®.
BrdU immunohistochemistryFor the detection of newly generated cells in the hippocampal dentate gyrus, BrdU-specific immunohistochemistry was performed as the previously described method (Kim et al., 2012). In brief, the brain sections were permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2 x standard saline citrate (SSC) at 65°C for 2 h, denaturated in 2 N HCl at 37°C for 30 min, and rinsed twice in 100 mM sodium borate (pH 8.5). Afterwards, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Vector Laboratories). The sections were then washed three times with PBS and incubated for 1 h with the biotinylated mouse secondary antibody (1:200; Vector Laboratories). Then the sections were incubated for another 1 h with avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, the sections were incubated for 5 min in 50 mM Tris-HCl (pH 7.6) containing 0.02% DAB, 40 mg/mL nickel chloride, and 0.03% hydrogen peroxide. Subsequently, the slides were air-dried overnight at room temperature, and coverslips were mounted using Permount®.
Data analysisAll values are expressed as the mean±standard error of the mean (SEM). For comparisons among the groups, one-way analysis of variance (ANOVA) and Duncan’s post-hoc test were performed with P<0.05 as an indication of statistical significance. The number of TH-immunoreactive neurons in the substantia nigra was counted in each section using a microscope (Olympus, Tokyo, Japan). The survival rate of TH-positive cells in the substantia nigra was calculated as follows: the number of TH-positive cells in the lesion side/the number of TH-positive cells in the intact side. To estimate the TH-staining density, the optical densities were corrected for nonspecific background density, which was measured in completely denervated parts of the striatum. The ratio of TH-positive fiber density in the striatum was calculated as follows: optical density in the lesion side/optical density in the intact side. The number of caspase-3-positive cells in the ipsilateral substantia nigra was counted in each section. The area of the granular layer of dentate gyrus was traced using Image-Pro®Plus image analyzer (Media Cybernetics Inc., Silver Spring, MD, USA) at 40×magnification. The number of BrdU-positive cells was expressed as the mean number of cells per mm2 of the cross sectional area of the granular layer of the dentate gyrus.
RESULTSEffect of 6-OHDA treatment on apomorphine-induced rotation testAssessment of apomorphine-induced changes in rotational behavior at 4 weeks after injecting of 6-OHDA into the striatum was conducted. The number of net rotation was −15.05±11.4 turns/h in the sham-operation group and 108.75±21.8 turns/h in the 6-OHDA-injection group. Rotational asymmetry was induced 4 weeks after injection of 6-OHDA into the striatum.
Effect of treadmill exercise on latency in the step-down avoidance task after 6-OHDA-injectionThe latency in the step-down avoidance task was 230.56±26.95 sec in the sham-operation group and 235.33±25.85 sec in the sham-operation and exercise group. Treadmill exercise exerted no significant effect on the latency in normal rats. The latency in the 6-OHDA-injection group was 81.70±17.55 sec and 250.70±21.00 sec in the 6-OHDA-injection and exercise group. Short-term memory was significantly decreased without treadmill exercise for 2 weeks. However, treadmill exercise for 2 weeks protected 6-OHDA-induced decline of short-term memory (Fig. 1).
Effect of treadmill exercise on TH expression in the nigrostriatum after 6-OHDA injectionPhotomicrographs of TH-positive cells in the substantia nigra are presented in Fig. 2. Survival rate of TH-immunoreactive neurons in the substantia nigra was 94.73±0.80% in the sham-operation group, 94.86±4.80% in the sham-operation and exercise group, 27.04±13.60% in the 6-OHDA-injection group, and 44.55±6.10% in the 6-OHDA-injection and exercise group. Survival rate of dopaminergic neurons in the substantia nigra was decreased without treadmill exercise for 2 weeks. However, tread-mill exercise for 2 weeks alleviated 6-OHDA-induced dopaminergic neuronal cell death in the substantia nigra.
Photomicrographs of TH-immunoreactive fibers in the striatum are presented in Fig. 3. The optical density of TH-immunoreactive fibers in the striatum was 0.99±0.02 in the sham-operation group, 1.03±0.01 in the exercise group, 0.70±0.02 in the 6-OHDA-injection group, and 0.80±0.05 in the 6-OHDA-injection and exercise group. Fiber density of dopaminergic neurons in the striatum was decreased without treadmill exercise for 2 weeks. However, treadmill exercise for 2 weeks alleviated 6-OHDA-induced dopaminergic fiber loss in the striatum.
Effect of treadmill exercise on caspase-3 expression in the substantia nigra after 6-OHDA injectionPhotomicrographs of caspase-3-positive cells in the substantia nigra are presented in Fig. 4. The number of caspase-3-positive cells was 25.20±4.08/section in the sham-operation group and 22.89±5.51/section in the sham-operation and exercise group. There was no statistically significant difference in the number of caspase-3-positive cells between the sham-operation group and the sham-operation and exercise group. The number of caspase-3-positive cells was increased to 58.60±12.14/section in the 6-OHDA-injection group, but it was reduced to 44.36±18.81/section in the 6-OHDA-injection and exercise group. Caspase-3 expression in the substantia nigra was increased without treadmill exercise for 2 weeks. However, treadmill exercise for 2 weeks suppressed the 6-OHDA-induced caspase-3 expression in the substantia nigra.
Effect of treadmill exercise on cell proliferation in the dentate gyrus after 6-OHDA injectionPhotomicrographs of BrdU-positive cells in the dentate gyrus are presented in Fig. 5. The number of BrdU-positive cells was 305.83±14.33/mm2 in the sham-operation group, 396.79±18.19/mm2 in the sham-operation and exercise group, 214.18±13.11/mm2 in the 6-OHDA-injection group, and 407.00±22.79/mm2 in the 6-OHDA-injection and exercise group. New cell proliferation in the dentate gyrus was suppressed without treadmill exercise for 2 weeks. However, treadmill exercise for 2 weeks enhanced new cell proliferation in the dentate gyrus under both normal and Parkinson’s disease conditions.
DISCUSSIONCaspase-3 is a critical factor for cell death in the substantia nigra of Parkinson’s patients, and it is the initiating caspase in Parkinson’s disease (Andersen, 2001; Hartmann et al., 2000). Pathogenesis of Parkinson’s disease involves glutamate toxicity and mitochondrial defects (Schapira, 2001). 6-OHDA induces oxidative stress (Jakel et al., 2005) and injection of 6-OHDA initiated apoptotic neuronal cell death in the substantia nigra and decreased the number of dopaminergic neurons (Blum et al., 2001; Yoon et al., 2007). In the present results, 6-OHDA injection into the striatum, an extensive degeneration of the dopaminergic neurons in the substantia nigra with loss of dopaminergic fibers in the striatum was produced, which resulted in short-term memory impairment. Apoptotic neuronal cell death in the substantia nigra was also occurred by intrastriatal injection of 6-OHDA.
Physical exercise attenuates behavioral and neurobiological abnormalities of the 6-OHDA-treated rats (Cohen et al., 2003; Tillerson et al., 2003). Cohen et al. (2003) observed that exercise increased glial cell-line neurotrophic factor (GDNF) expression in the striatum after 6-OHDA injection. GDNF possesses protective and restorative effects against 6-OHDA-induced neurotoxicity in the dopaminergic neurons. Exercise decreased the ratio between dopamine transporter and vesicular monoamine transporter, which might lower the susceptibility of dopaminergic neurons to neurotoxins and reduce cytosolic dopamine oxidation (Tillerson et al., 2003). Faherty et al. (2005) showed that environmental enrichment including wheel running reduced 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced degeneration of dopaminergic neurons in mice via increasing in GDNF expression. Physical exercise is known to activate endogenous anti-oxidant systems in the brain and down-regulate the expression of glutamate receptors implicated in excitotoxicity (Devi et al., 2004). Treadmill exercise ameliorated MPTP-induced dopaminergic neuronal loss by inhibiting brain inflammation in mice (Sung et al., 2012). In the present results, treadmill exercise for 2 weeks enhanced survival of nigrostriatal dopaminergic neurons and decreased apoptotic cell death in the substantia nigra of 6-OHDA-induced Parkinson’s rats.
Hippocampal atrophy correlates with memory impairment in Parkinson’s patients, and dopaminergic dysfunction may disturb spatial working memory and attentional set-shifting accuracy (Riekkinen et al., 1998). Cell proliferation in the subgranular zone of the dentate gyrus may be under the dopaminergic control, and the number of neural precursors in the dentate gyrus was decreased in the MPTP-treated mice with Parkinson’s disease (Honglinger et al., 2004). In present results, cell proliferation in the dentate gyrus was suppressed and short-term memory was deteriorated by intrastriatal injection of 6-OHDA.
Exercise increases cell proliferation in the hippocampal dentate gyrus and enhances long-term potentiation, spatial learning, synaptic strength, and memory functions (Kim et al., 2004; van Praag et al., 1999). Generation of new neurons in the hippocampus is essential for the maintaining of normal learning and memory processes (Kim et al., 2012). In present results, treadmill exercise alleviated short-term memory impairment through increasing hippocampal cell proliferation in the 6-OHDA-induced Parkinson’s rats.
Here, we have shown that treadmill exercise reduced nigrostriatal dopaminergic neuronal cell death and fiber loss and increased cell proliferation in the 6-OHDA-induced Parkinson’s rats. These results suggest that treadmill exercise may provide therapeutic value for the treatment of Parkinson’s disease.
REFERENCESAndersen JK. Does neuronal loss in Parkinson’s disease involve programmed cell death? Bioessays. 2001;23:640–646.
Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson’s disease. Prog Neurobiol. 2001;65:135–172.
Camicioli R, Moore MM, Kinney A, Corbridge E, Glassberg K, Kaye JA. Parkinson’s disease is associated with hippocampal atrophy. Mov Disord. 2003;18:784–790.
Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669.
Cohen AD, Tillerson JL, Smith AD, Schallert T, Zigmond MJ. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305.
Devi SA, Kiran TR. Regional responses in antioxidant system to exercise training and dietary Vitamin E in aging rat brain. Neurobiol Aging. 2004;25:501–508.
Döbrössy MD, Dunnett SB. Motor training effects on recovery of function after striatal lesions and striatal grafts. Exp Neurol. 2003;184:274–284.
Dujardin K, Laurent B. Dysfunction of the human memory systems: role of the dopaminergic transmission. Curr Opin Neurol. 2003;16:S11–S16.
Eriksson PS, Perfilieva E, Bjök-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nat Med. 1998;11:1313–1317.
Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134:170–179.
Fuchs E, Gould E. Mini-review: in vivo neurogenesis in the adult brain: regulation and functional implications. Eur J Neurosci. 2000;12:2211–2214.
Hartmann A, Hunot S, Michel PP, Vyas S, Faucheux BA, Mouattprigent A, Turmel H, Srinivasn A, Ruberg M, Evan GI, Agid Y. Caspase-3: a Vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc Natl Acad Sci U S A. 2000;97:2875–2880.
He Y, Lee T, Leong SK. 6-Hydroxydopamine induced apoptosis of dopaminergic cells in the rat substantia nigra. Brain Res. 2000;858:163–166.
Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735.
Jakel RJ, Kern JT, Johnson DA, Johnson JA. Induction of the Protective Antioxidant Response Element Pathway by 6-Hydroxydopamine in vivo and in vitro. Toxicol Sci. 2005;87:176–186.
Jellinger K, Linert L, Kienzl E, Herlinger E, Youdim MB. Chemical evidence for 6-hydroxydopamine to be an endogenous toxic factor in the pathogenesis of Parkinson’s disease. J Neural Transm Suppl. 1995;46:297–314.
Kim JE, Ji ES, Seo JH, Lee MH, Cho S, Park YK, Seo TB, Kim CJ. Alcohol exposure induces depression-like behavior by decreasing hippocampal neuronal proliferation through inhibition of the BDNF-ERK pathway in gerbils. Anim Cells Syst. 2012;16:190–197.
Kim YP, Kim H, Shin MS, Chang HK, Jang MH, Shin MC, Lee SJ, Lee HH, Yoon JH, Jeong IG, Kim CJ. Age-dependence of the effect of treadmill exercise on cell proliferation in the dentate gyrus of rats. Neurosci Lett. 2004;355:142–154.
Kim YM, Ji ES, Yoon SJ, Yoon JW. Sudden detraining deteriorates swimming training-induced enhancement of short-term and spatial learning memories in mice. J Exer Rehabil. 2013;9:243–249.
Lee MH, Kim H, Kim SS, Lee TH, Lim BV, Chang HK, Jang MH, Shin MC, Shin MS, Kim CJ. Treadmill exercise suppresses ischemia-induced increment in apoptosis and cell proliferation in hippocampal dentate gyrus of gerbils. Life Sci. 2003;73:2455–2465.
Lev N, Melamed E, Offen D. Apoptosis and Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:245–250.
Miyai I, Fujimoto Y, Ueda Y, Yamamoto H, Nozaki S, Saito T, Kang J. Treadmill training with body weight support: its effect on Parkinson’s disease. Arch Phys Med Rehabil. 2000;81:849–852.
Riekkinen P Jr, Kejonen K, Laakso MP, Soininen H, Partanen K, Riekkinen M. Hippocampal atrophy is related to impaired memory, but not frontal junctions in non-demented parkinson’s disease patients. Neuroreport. 1998;9:1507–1511.
Schalow G, Paasuke M, Jaigma P. Integrative re-organization mechanism for reducing tremor in Parkinson’s disease patients. Electromyogr Clinl Neurophysiol. 2005;45:407–415.
Sung YH, Kim SC, Hong HP, Park CY, Shin MS, Kim CJ, Seo JH, Kim DY, Kim DJ, Cho HJ. Treadmill exercise ameliorates dopaminergic neuronal loss through suppressing microglial activation in Parkinson’s disease mice. Life Sci. 2012;91:1309–1316.
Tillerson JL, Caudle WM, Reveron ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson’s disease. Neuroscience. 2003;119:899–911.
Trejo JL, Carro E, Torres-Aleman I. Circulating insulin-like growth factor I mediates exercise-induced increases in the number of new neurons in the adult hippocampus. J Neurosci. 2001;21:1628–1634.
Fig. 1.Effect of treadmill exercise on latency in the step-down avoidance task. Values are presented as the mean ± standard error of the mean (SEM). (A) Sham-operation group, (B) sham-operation and exercise group, (C) 6-OHDA-injection group, (D) 6-OHDA-injection and exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P< 0.05 compared to the 6-hydroxydopamine (6-OHDA)-injection group. 
Fig. 2.Effect of treadmill exercise on tyrosine hydroxylase (TH) expression in the substantia nigra. Upper: Photomicrographs showing TH-specific immunohistochemical staining of the substantia nigra. The scale bar represents 200 μm. Lower: Survival rates of TH-immunoreactive neurons in each group. Values are presented as the mean±standard error of the mean (SEM). (A) Sham-operation group, (B) sham-operation and exercise group, (C) 6-OHDA-injection group, (D) 6-OHDA-injection and exercise group. *represents P<0.05 compared to the sham-operation group. #represents P< 0.05 compared to the 6-hydroxydopa-mine (6-OHDA)-injection group. 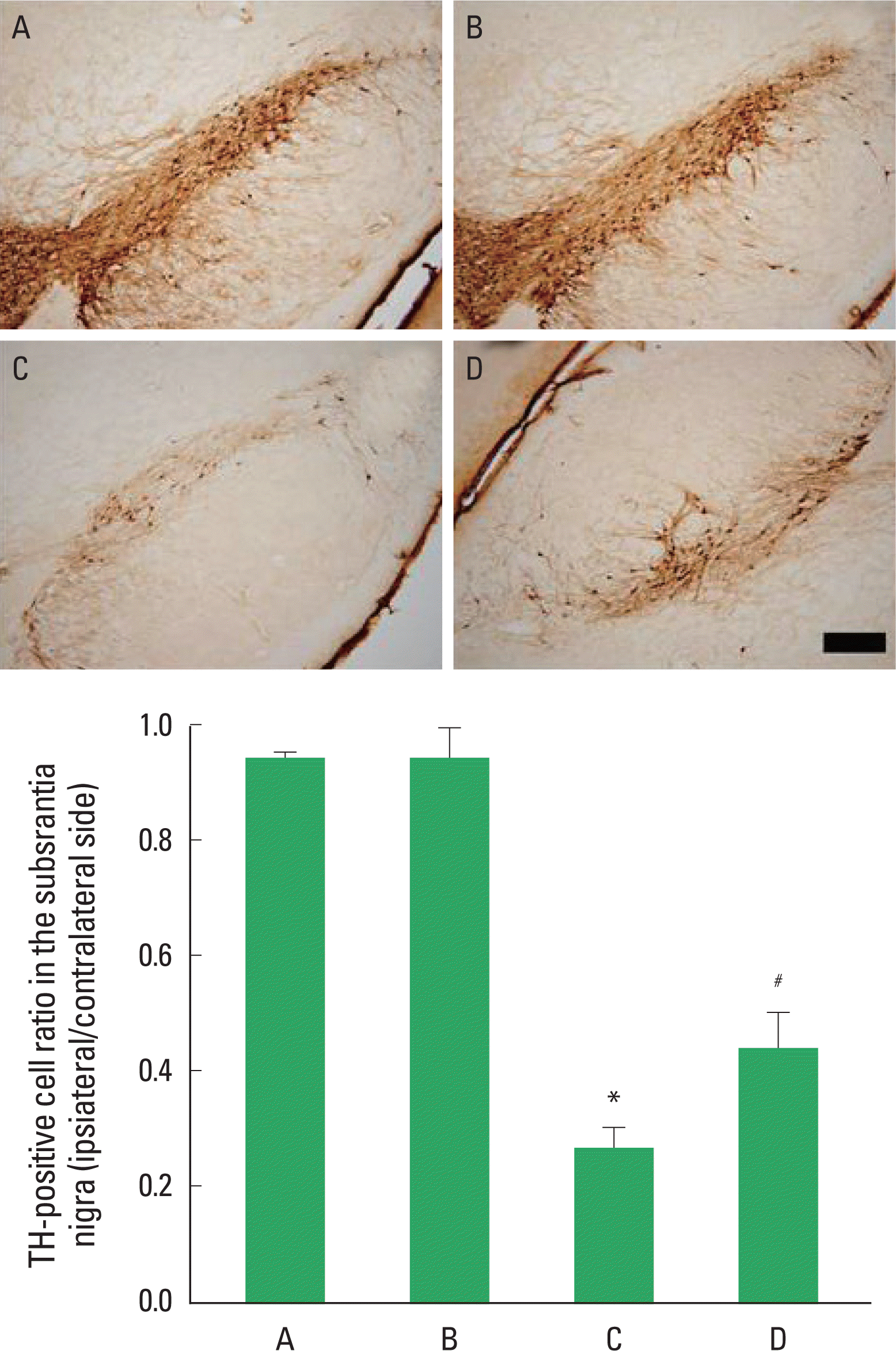
Fig. 3.Effect of treadmill exercise on tyrosine hydroxylase (TH) expression in the striatum. Upper: Photomicrographs of TH-positive fibers in the striatum. The scale bar represents 500 μm. Lower: The optical density of TH-immunore-active fibers. Values are presented as the mean ± standard error of the mean (SEM). (A) Sham-operation group, (B) sham-operation and exercise group, (C) 6-OHDA-injection group, (D) 6-OHDA-injection and exercise group. *represents P<0.05 compared to the sham-operation group. #represents P<0.05 compared to the 6-hydroxydopamine (6-OHDA)-injection group. 
Fig. 4.Effect of treadmill exercise on caspase-3 expression in the substantia nigra. Upper: Photomicrographs of in the substantia nigra. The scale bar represents 200 μm. Lower: The number of caspase-3-positive cells. Values are presented as the mean±standard error of the mean (SEM). (A) Sham-operation group, (B) sham-operation and exercise group, (C) 6-OHDA-injection group, (D) 6-OHDA-injection and exercise group. *represents P<0.05 compared to the sham-operation group. #represents P<0.05 compared to the 6-hydroxydopamine (6-OHDA)-injection group. 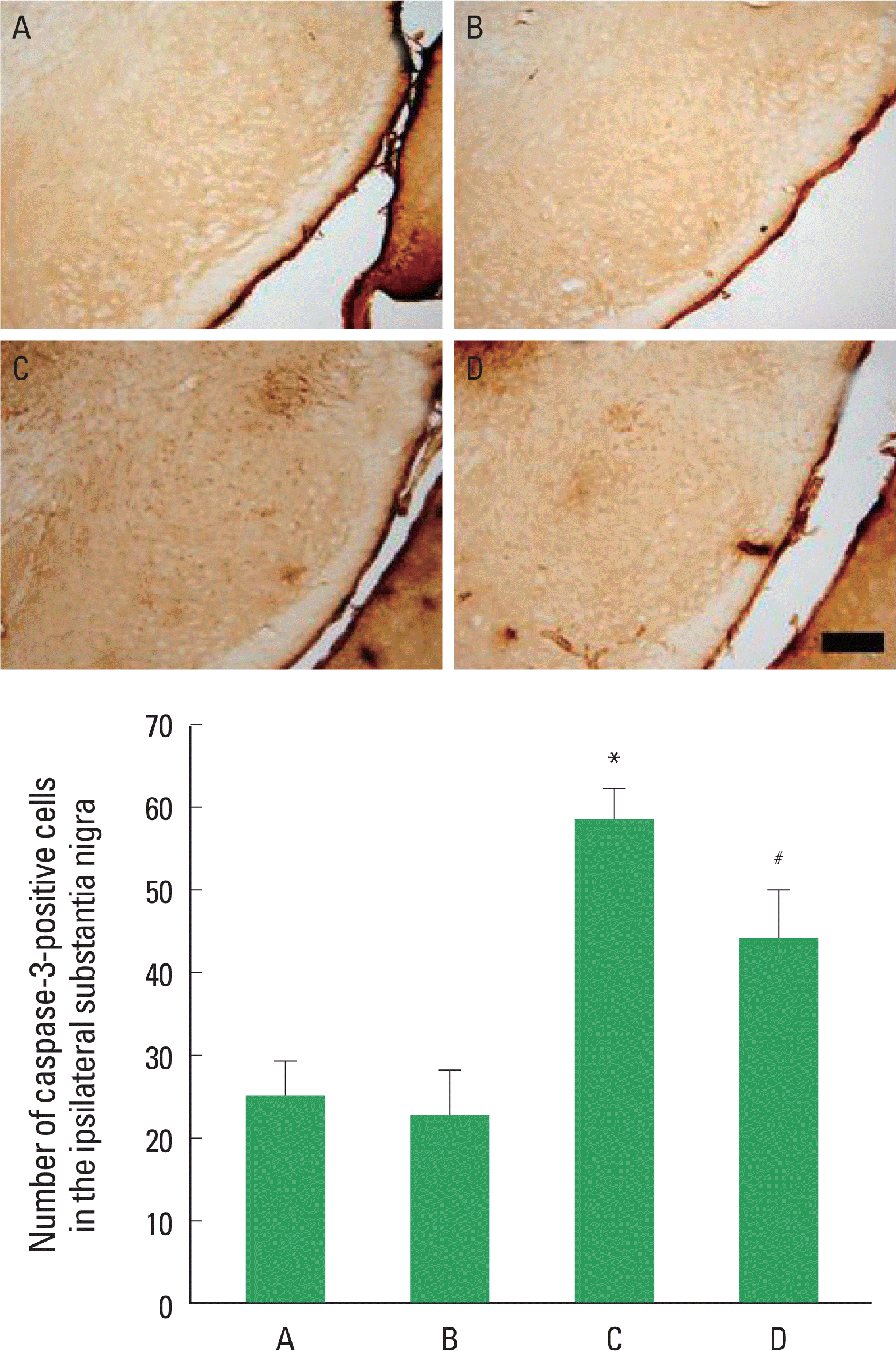
Fig. 5.Effect of treadmill exercise on cell proliferation in the hippocampal den-tate gyrus. Upper: Photomicrographs of 5-bromo-2′-deoxyuridine (BrdU)-positive cells. The scale bar represents 200 μm. Lower: The number of BrdU-positive cells. Values are presented as the mean±standard error of the mean (SEM). (A) Sham-operation group, (B) sham-operation and exercise group, (C) 6-OHDA-injection group, (D) 6-OHDA-injection and exercise group. *represents P< 0.05 compared to the sham-operation group. #represents P< 0.05 compared to the 6-hydroxydopamine (6-OHDA)-injection group. 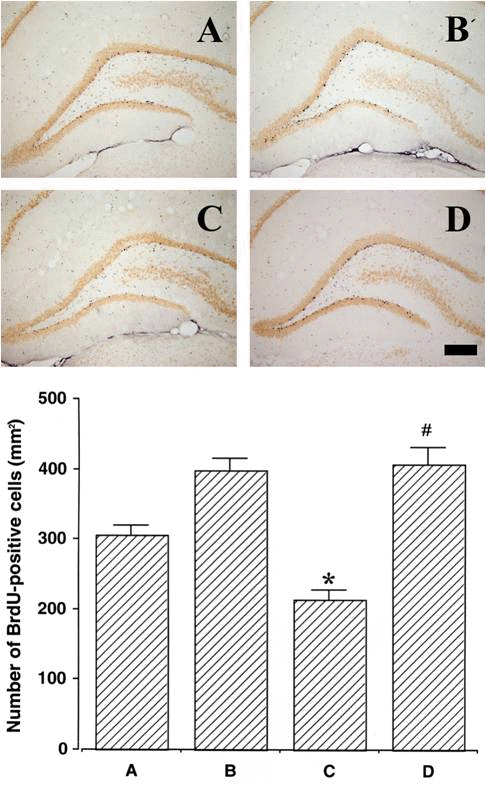
|
|
|||||||||||||||||||||||||||||||||||