AbstractSchizophrenia is a serious psychiatric disorder with several symptoms including cognitive dysfunction. Although the causes of schizophrenia are still unclear, there is a strong suspicion that the abnormality in N-methyl-D-aspartate (NMDA) receptor may contribute to schizophrenia symptoms. In the present study, the effect of treadmill exercise on the NMDA receptor expression was evaluated using MK-801-induced schizophrenia mice. Immunohistochemistry for expressions of NMDA receptor tyrosine hydroxylase (TH) was conducted. Western blot for brain-derived neurotrophic factor (BDNF) was also performed. In the present results, the mice in the MK-801-treated group displayed reduced NMDA receptor expression. Enhanced TH expression and suppressed BDNF expression were also observed in the MK-801-treated mice. Treadmill exercise improved NMDA receptor expression in the MK-801-induced schizophrenia mice. Treadmill exercise also suppressed TH expression and enhanced BDNF expression in the MK-801-induced schizophrenia mice. The present study showed that down-regulation of NMDA receptor demonstrated schizophrenia-like parameters, meanwhile treadmill running improved schizophrenia-related parameters through enhancing NMDA receptor expression.
INTRODUCTIONSchizophrenia is a serious psychiatric disorder with 0.5 to 1.0 percent prevalence worldwide (Ross et al., 2006). Schizophrenia causes particular collapse in personality, such as thought without any clouding of consciousness, affection, perception, volition, and motor behavior, etc. The exact mechanisms of schizophrenia have not been identified, however previous studies have suggested that neurochemical dysfunction is important clue for schizophrenia, such as dopamine dysfunction, dopamine-glutamate imbalance, and glutamate receptor hypofunction (Carlsson et al., 1990, 1999; Deutsch et al., 2002). According to the Coyle (2006), there is new evidence to suspect that the abnormality in NMDA receptor function may contribute to schizophrenia’s symptoms that are resistant to antipsychotic medication.
Glutamate is the primary excitatory neurotransmitter in the mammalian brain. Glutamate acts postsynaptically on the three families of ionotropic receptors, such as N-methyl D-aspartate (NMDA) receptor, a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor, and kainate receptor (McBain and Mayer, 1994). Excitatory action of glutamate is prominent particularly in the hippocampus, out layer of the cerebral cortex, and substantia gelationsa of the spinal cord. Excitatory action of glutamate is implicated in many physiological processes including learning and memory (Hudspith. 1997).
Dopamine is synthesized first by hydroxylation of the amino acid L-tyrosine to 3,4-dihydroxy-L-phenylalanine by the enzyme tyrosine hydroxylase (TH), and then it is decarboxylated by L-amino acid decaboxylase to dopamine (Dunkley et al., 2004).
Brain-derived neurotrophic factor (BDNF) is one of the neurotrophin families which interact with high affinity protein tyrosine kinase receptor (Trk). BDNF plays key roles in early embryogenensis by enhancing the differentiation of neuronal precursors as well as being a critical component of early neural tube development in the chick (Kernie et al., 2000). BDNF expression is altered in various brain diseases, and BDNF is also involved in eating behavior and physical activity (Trpia-Arancibia et al., 2004).
Schizophrenia patients show deficit in a variety of cognitive function, and this deficit is associated with dysfunction of network in brain areas including the frontal and temporal cortex, the hippocampus, and the subcortial regions (Kuperberg and Heckers, 2000). In particularly, dysfunction of prefrontal cortex is associated with working memory deficit (Manoach. 2003), and this abnormality is predominant in schizophrenia (Meyer-Lindenberg et al., 2002).
Administration of NMDA receptor antagonists and dopaminergic agonists to animals is the most widely used animal models for schizophrenia (Rung et al., 2005). MK-801, also known as dizocilpine maleate, is a non-competitive NMDA receptor antagonist, and this agent has been used for inducing schizophrenia-like symptoms in animals.
Exercise is known to protect neurons from various brain insults, activates neuronal cells, promotes neurogenesis, increases brain plasticity, and enhances cognitive function (Collins et al., 2009; van Praag, 2009; Weuve et al., 2004). Many studies have revealed that exercise improves symptoms of neuropsychiatric disorders (Cho et al., 2013; Jee et al., 2008; Seo et al., 2013), however the effect of treadmill exercise on the schizophrenia in relation with NMDA receptor expression have not been evaluated. In the present studies, the effect treadmill running on the NMDA receptor expression was investigated using MK-801-induced schizophrenia mice.
MATERIALS AND METHODSMaterialsMK-801 was dissolved in saline (0.9% NaCl solution) as master stock solution (1 mg/mL). The stock solution was diluted with saline to the final concentrations (0.6 mg/kg) for systemic administration by intraperitoneal injection. Dilution was designed to give a final injection volume of 100 μL per 10 grams of animal weight for each injection of MK-801.
Experimental animals and designMale C57BL/6 mice weighing 27±3 g (6 weeks of age) were used for this experiment. The animals were housed under standard housing conditions with plastic cages. The animals were housed under controlled temperature (23±2°C) and lighting (08:00 to 20:00) conditions, and were supplied with food and water ad libitum. The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health (NIH) and the Korean Academy of Medical Sciences. The mice were randomly divided into three groups (n=10 in each group): control group, MK-801 injection group, MK-801 injection and treadmill exercise group.
Exercise protocolThe mice in the treadmill exercise group were made to run on a motor-driven treadmill for 1 hour once a day during 2 weeks. The exercise load consisted of running at the speed of 2 meters/min for the first 5 min, 5 meters/min for the next 5 min, and then 8 meters/min for the last 50 min, with the 0° inclination. The mice in the control group and in the MK-801 injection group were left in the treadmill, without running, for the same duration as the exercise group.
Tissue preparationThe mice were sacrificed immediately after last treadmill exercise. The animals were anesthetized using Zoletil 50® (10 mg/kg, i.p.; Vibac Laboratories, Carros, France), transcardially perfused with 50 mM phophate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100 mM phophate buffer (PB, pH 7.4). Brain was dissected, post-fixed in the same fixative overnight and tranferred to 30% sucrose for cryoprotection. 40 μm thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany). Ten slice sections on average were collected from each mouse.
NMDA receptors immunohistochemistryTo visualize NMDA receptor expression, immunohistochemistry for NMDA receptor was performed, as the previously described method (Eleore et al., 2005). The sections were selected from each brain and were incubated overnight with anti-NMDR1 primary antibody (1:1,000; Vector Laboratories, Burlingame, CA, USA), and then with biotinylated second antibody for 1 h. The second antibody was amplified with the Vector Elite ABD kit® (1:100; Vector Laboratories). Anti-biotin-avidin-peroxidase complexes was visualized using 0.03% 3,3-diaminobenzidine (DAB), and the section was mounted onto gleatin-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific, Fair Lawn, NJ, USA).
Tyrosine hydroxylase immunohistochemistryFor immunolabeling of TH in the striatum, TH immunohistochemistry was performed as the previously described method (Cho et al., 2013; Kim et al., 2005). Free-floating tissue sections were incubated overnight with mouse anti-TH antibody (1:1,000, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and the sections were then incubated for 1 h with biotinylated anti-mouse secondary antibody (1:2,000, Vector Laboratories). The sections were subsequently incubated with avidin-biotin-peroxidase complex (Vector Laboratories) for 1 h at room temperature. Immunoreactivity was visualized by incubating the sections in a solution consisting of 0.05% DAB and 0.01% H2O2 in 50 mM Tris-buffer (pH 7.6) for approximately 3 min. The sections were then washed three times with PBS and mounted onto gelatine-coated slides. The slides were air-dried overnight at room temperature, and coverslips were mounted using Permount® (Fisher Scientific).
Western blot for BDNF expressionBDNF expression was determined by western blot as the previously described method (Kim et al., 2010). The hippocampal tissues were collected, and then are immediately frozen at −70°C. The hippocampal tissues was homogenized on ice, and lysed in a lysis buffer containing 50 mM Tris-HCl (pH 7.5), 150 mM NaCl, 0.5% deoxycholic acid, 1% Nonidet P40, 0.1% SDS, 1 mM PMSF, and 100 mg/mL leupeptin. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Bio-Rad, Hercules, CA, USA). Protein of 30 μg was separated on SDS-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse β actin antibody (1:3,000; Santa Cruz Biotechnology), rabbit BDNF antibody (1:1,000; Santa Cruz Biotechnology).
Horseradish peroxidase-conjugated anti-rabbit antibody for BDNF was used as the secondary antibody. Experiment was performed in normal lab conditions and at room temperature except for transferred membrane. Membrane transfer was performed at 4°C with the cold pack and prechilled buffer. Band detection was performed using the enhanced chemiluminescence (ECL) detection kit (Santa Cruz Biotechnology). To compare relative expression of proteins, detected bands were calculated densitometrically using Molecular Analyst™ version1.4.1 (Bio-Rad).
Data analysisFor confirming the expression of BDNF, the detected bands were calculated densitometrically using Molecular Analyst™, version 1.4.1. The number of NMDA-positive cells were counted hemi-laterally under a light microscope (Olympus, Tokyo, Japan), and they were expressed as the number of cells per square millimeter. The area of the dentate gyrus was measured by Image-Pro® Plus image analysis system (Media Cyberbetics Inc., Silver Sprng, MD, USA). To estimate the TH-staining density, the optical densities were corrected for nonspecific background density, which was measured in completely denervated parts of the striatum. The ratio of TH-positive fiber density in the striatum was calculated as follows: optical density in the lesion side/optical density in the intact side.
All data were analyzed using the statistical software SPSS (version 15.0). The data was analyzed with one-way ANOVA and then Duncan’s post-hoc tests. All values are expressed as the mean±standard error of the mean (SEM). The P value<0.05 was considered significant.
RESULTSEffect of treadmill exercise on the number of NMDA receptor-positive cells in the dentate gyrusPhotomicrographs of the NMDA receptor-positive cells in the dentate gyrus are presented Fig. 1. The number of NMDA receptors-positive cells was 220.40±18.91/section in the control group, 64.01±7.93/section in the MK-801 injection group, and 275.92±24.74/section in the MK-801 injection and treadmill exercise group. The present results showed that the number of NMDA receptor-positive cells in the dentate gyrus of the MK-801 injection group was decreased compared to the control group (P<0.05). In contrast, the number of NMDA receptor-positive cells in the dentate gyrus of the treadmill exercise group was increased (P<0.05).
Effect of treadmill exercise on the number of NMDA receptor-positive cells in the prefrontal cortexPhotomicrographs of the NMDA receptor-positive cells in the prefrontal cortex are presented Fig. 2. The number of NMDA receptors-positive cells were 1,724.10±47.50/section in the control group, 1,022.50±28.03/section in the MK-801 injection group, 1,355.43±22.48/section in the MK-801 injection and treadmill exercise group. The present results showed that the number of NMDA receptor-positive cells in the prefrontal cortex of the MK-801 injection group was decreased compared to the control group (P<0.05). In contrast, the number of NMDA receptor-positive cells in the prefrontal cortex of the treadmill exercise group was increased (P<0.05).
Effect of treadmill exercise on the TH expression in the striatumPhotomicrographs of the TH in the striatum are presented Fig. 3. The density of TH was 87.66±3.22 in the control group, 104.41±3.24 in the MK-801 injection group, 89.90±0.79 in the MK-801 injection and treadmill exercise group. The present result showed that the TH expression in the striatum of the MK-801 injection group was increased compared to the control group (P<0.05). In contrast, TH expression in the striatum of the treadmill exercise group was increased (P<0.05).
Effects of treadmill exercise on the BDNF expression in the hippocampusThe BDNF (14 kDa) protein expressions in the hippocampus are presented Fig. 4. When the level of BDNF in the control group was set at 1.00, the level of BDNF was 0.77±0.03 in the MK-801 injection group, 0.97±0.01 in the MK-801 injection and treadmill exercise group. The present result showed that the expression of BDNF in the hippocampus of the MK-801 injection group was decreased in compared to the control group (P<0.05). In contrast, BDNF expression in the hippocampus of the treadmill exercise group was increased (P<0.05).
DISCUSSIONMany studies have indicated that NMDA receptor hypofunction may contribute to the pathophysiology of schizophrenia and induces cognitive and behavior abnormalities (Duncan et al., 2004; Kristiansen et al., 2007; Newcomer and Krystal, 2001). NMDA receptor causes diverse functions in the sensory and motor system (Daw et al., 1993). Duncan et al. (2006) reported that NMDA receptor hypofunction is related to the increased locomotor activity. NMDA receptor antagonists induce hyperactivity that resembles schizophrenia-like symptom (Leriche et al., 2003). Mice with reduced NMDA receptor expression display behavioral abnormalities, including deficit in social interaction (Mohn et al., 1999). Postmortem study reported that NMDA receptor subunit was decreased in the brain regions of schizophrenia patients, especially in the hippocampus and prefrontal cortex (Harrison et al., 2003).
In the present results, NMDA receptor expression was suppressed in the prefrontal cortex and hippocampus of the MK-801-iduced schizophrenia mice. The present results also showed that treadmill exercise increased NMDA receptor expression in the hippocampus and prefrontal cortex.
Previous studies revealed that exercise lead to change in NMDA receptor expression. Kitamura et al. (2003) reported that wheel exercise may activate NMDA receptors in the hippocampus, and exercise increased the phosphorylated form of NMDA receptor (Dietrich et al., 2005). NMDA receptor exerts an important role in neuronal plasticity and synaptic signaling through a fine control of its channel activity (Dietrich et al., 2005). Exercise also activates NMDA receptor in the hippocampus (Dietrich et al., 2005; Vaynman et al., 2003).
Zafra et al. (1990) suggested that the synthesis of neurotrophic factors such as BDNF and nerve growth factor (NGF) in the brain is regulated by neuronal activity via NMDA receptor. BDNF expression was decreased in the several neuropsychiatric disorders, including schizophrenia and depression (Knable et al., 2004). NMDA receptor antagonist MK-801 reduced expression of BDNF in the hippocampus (Fumagalli et al., 2003).
In the present study, BDNF expression was decreased hippocampus of the MK-801-iduced schizophrenia mice. The present results also showed that treadmill exercise increased BDNF expression in the hippocampus.
BDNF-improving effect of physical exercise has been reported in many other studies (Garza et al., 2004; Griebach et al., 2004; Huang et al., 2006). Exercise-induced enhancement of BDNF expression in the hippocampus inhibited age-dependent deterioration of short-term and spatial working memories (Kim et al., 2010). High level of BDNF also alleviates spatial working memory impairment in attention deficit/hyperactivity disorder (ADHD) rats (Kim et al., 2011).
NMDA receptor antagonists, such as MK-801 or phencyclidine induced behavioral abnormalities which are accompanied by an increment in dopaminergic and serotonergic neuronal activity in various brain regions (Löscher et al., 1991). The glutamatergic system and dopaminergic system are tightly linked to each other. Reduced glutamate function, induced by MK-801, may cause some elevation of dopamine release (Carlsson et al., 2000). Larulle et al. (2003) reported that deficits in glutamate transmission might lead to the increment of dopamminergic biological marker associated with schizophrenia. TH is the first and rate-limiting enzyme in catecholamine synthesis and it catalyses the hydroxylation of l-tyrosine to DOPA (Dunkley et al., 2004). TH activity is known to be gradually decreased following the loss of dopamine neurons (Yoon et al., 2007), as a result, TH represents the rate of dopamine synthesis (Howes et al., 2009).
The present results showed that the density of TH in the striatum was increased by MK-801 injection. Dopamine hyperactivity in the striatum is associated with the prodromal signs of schizophrenia (Howes et al., 2009). In the present results, treadmill exercise suppressed overactivity of density of TH. Lindgren et al. (2000) observed that NMDA receptor activity decreased dopamine synthesis in the stiatum. As mentioned above, it is possible that the suppression of TH is due to the increment of NMDA receptor by exercise.
The present study showed that down-regulation of NMDA receptor demonstrated schizophrenia-like parameters, meanwhile treadmill running improved schizophrenia-related parameters through enhancing NMDA receptor expression. Based on the present results, it can be suggested that treadmill exercise may be used as the one of the important strategies for the prevention and treatment of schizophrenia patients.
REFERENCESCarlsson A, Waters N, Carlsson ML. Neurotransmitter interactions in schizophrenia--therapeutic implications. Biol Psychiatry. 1999;46:1388–1395.
Carlsson A, Waters N, Waters S, Carlsson ML. Network interactions in schizophrenia - therapeutic implications. Brain Res Brain Res Rev. 2000;31:342–359.
Cho HS, Shin MS, Song W, Jun TW, Lim BV, Kim YP, Kim CJ. Treadmill exercise alleviates short-term memory impairment in 6-hydroxydopamine-induced Parkinson’s rats. J Exerc Rehabil. 2013;9:354–361.
Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379.
Collins A, Hill LE, Chandramohan Y, Whitcomb D, Droste SK, Reul JM. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS One. 2009;4:e4330
Coyle JT. Glutamate and schizophrenia: beyond the dopamine hypothesis. Cell Mol Neurobiol. 2006;26:365–384.
Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–222.
Deutsch SI, Rosse RB, Billingslea EN, Bellack AS, Mastropaolo J. Topiramate antagonizes MK-801 in an animal model of schizophrenia. Eur J Pharmacol. 2002;2. 449:121–125.
Dietrich MO, Mantese CE, Porciuncula LO, Ghisleni G, Vinade L, Souza DO, Portela LV. Exercise affects glutamate receptors in postsynaptic densities from cortical mice brain. Brain Res. 2005;1065:20–25.
Duncan GE, Moy SS, Lieberman JA, Koller BH. Typical and atypical antipsychotic drug effects on locomotor hyperactivity and deficits in sensorimotor gating in a genetic model of NMDA receptor hypofunction. Pharmacol Biochem Behav. 2006;85:481–491.
Duncan GE, Moy SS, Perez A, Eddy DM, Zinzow WM, Lieberman JA, Snouwaert JN, Koller BH. Deficits in sensorimotor gating and tests of social behavior in a genetic model of reduced NMDA receptor function. Behav Brain Res. 2004;153:507–519.
Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;1025–1043.
Eleore L, Vassias I, Vidal PP, de Waele C. Modulation of the glutamatergic receptors (AMPA and NMDA) and of glutamate vesicular transporter 2 in the rat facial nucleus after axotomy. Neuroscience. 2005;136:147–160.
Fumagalli F, Molteni R, Roceri M, Bedogni F, Santero R, Fossati C, Gennarelli M, Racagni G, Riva MA. Effect of antipsychotic drugs on brain-derived neurotrophic factor expression under reduced N-methyl-D-aspartate receptor activity. J Neurosci Res. 2003;72:622–628.
Garza AA, Ha TG, Garcia C, Chen MJ, Russo-Neustadt AA. Exercise, antidepressant treatment, and BDNF mRNA expression in the aging brain. Pharmacol Biochem Behav. 2004;77:209–220.
Griesbach GS, Hovda DA, Molteni R, Wu A, Gomez-Pinilla F. Voluntary exercise following traumatic brain injury: brain-derived neurotrophic factor upregulation and recovery of function. Neuroscience. 2004;125:129–139.
Harrison PJ, Law AJ, Eastwood SL. Glutamate receptors and transporters in the hippocampus in schizophrenia. Ann N Y Acad Sci. 2003;1003:94–101.
Howes OD, Montgomery AJ, Asselin MC, Murray RM, Valli I, Tabraham P, Bramon-Bosch E, Valmaggia L, Johns L, Broome M, McGuire PK, Grasby PM. Elevated striatal dopamine function linked to prodromal signs of schizophrenia. Arch Gen Psychiatry. 2009;66:13–20.
Huang AM, Jen CJ, Chen HF, Yu L, Kuo YM, Chen HI. Compulsive exercise acutely upregulates rat hippocampal brain-derived neurotrophic factor. J Neural Transm. 2006;113:803–811.
Hudspith MJ. Glutamate: a role in normal brain function, anaesthesia, analgesia and CNS injury. Br J Anaesth. 1997;78:731–747.
Jee YS, Ko IG, Sung YH, Lee JW, Kim YS, Kim SE, Kim BK, Seo JH, Shin MS, Lee HH, Cho HJ, Kim CJ. Effects of treadmill exercise on memory and c-Fos expression in the hippocampus of the rats with intracerebroventricular injection of streptozotocin. Neurosci Lett. 2008;443:188–192.
Kernie SG, Liebl DJ, Parada LF. BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 2000;19:1290–1300.
Kim H, Heo HI, Kim DH, Ko IG, Lee SS, Kim SE, Kim BK, Kim TW, Ji ES, Kim JD, Shin MS, Choi YW, Kim CJ. Treadmill exercise and methylphenidate ameliorate symptoms of attention deficit/hyperactivity disorder through enhancing dopamine synthesis and brain-derived neurotrophic factor expression in spontaneous hypertensive rats. Neurosci Lett. 2011;504:35–39.
Kim SE, Ko IG, Kim BK, Shin MS, Cho S, Kim CJ, Kim SH, Baek SS, Lee EK, Jee YS. Treadmill exercise prevents aging-induced failure of memory through an increase in neurogenesis and suppression of apoptosis in rat hippocampus. Exp Gerontol. 2010;45:357–365.
Kim YK, Lim HH, Song YK, Lee HH, Lim S, Han SM, Kim CJ. Effect of acupuncture on 6-hydroxydopamine-induced nigrostratal dopaminergic neuronal cell death in rats. Neurosci Lett. 2005;384:133–138.
Kitamura T, Mishina M, Sugiyama H. Enhancement of neurogenesis by running wheel exercises is suppressed in mice lacking NMDA receptor epsilon 1 subunit. Neurosci Res. 2003;47:55–63.
Knable MB, Barci BM, Webster MJ, Meador-Woodruff J, Torrey EF. Molecular abnormalities of the hippocampus in severe psychiatric illness: postmortem findings from the Stanley Neuropathology Consortium. Mol Psychiatry. 2004;9:609–620.
Kristiansen LV, Huerta I, Beneyto M, Meador-Woodruff JH. NMDA receptors and schizophrenia. Curr Opin Pharmacol. 2007;7:48–55.
Kuperberg G, Heckers S. Schizophrenia and cognitive function. Curr Opin Neurobiol. 2000;10:205–210.
Laruelle M, Kegeles LS, Abi-Dargham A. Glutamate, dopamine, and schizophrenia: from pathophysiology to treatment. Ann N Y Acad Sci. 2003;1003:138–158.
Leriche L, Schwartz JC, Sokoloff P. The dopamine D3 receptor mediates locomotor hyperactivity induced by NMDA receptor blockade. Neuropharmacology. 2003;45:174–181.
Lindgren N, Xu ZQ, Lindskog M, Herrera-Marschitz M, Goiny M, Haycock J, Goldstein M, Hökfelt T, Fisone G. Regulation of tyrosine hydroxylase activity and phosphorylation at Ser (19) and Ser (40) via activation of glutamate NMDA receptors in rat striatum. J Neurochem. 2000;74:2470–2477.
Löscher W, Annies R, Hönack D. The N-methyl-D-aspartate receptor antagonist MK-801 induces increases in dopamine and serotonin metabolism in several brain regions of rats. Neurosci Lett. 1991;128:191–194.
Manoach DS. Prefrontal cortex dysfunction during working memory performance in schizophrenia: reconciling discrepant findings. Schizophr Res. 2003;60:285–298.
McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiol Rev. 1994;74:723–760.
Meyer-Lindenberg A, Miletich RS, Kohn PD, Esposito G, Carson RE, Quarantelli M, Weinberger DR, Berman KF. Reduced prefrontal activity predicts exaggerated striatal dopaminergic function in schizophrenia. Nat Neurosci. 2002;5:267–271.
Mohn AR, Gainetdinov RR, Caron MG, Koller BH. Mice with reduced NMDA receptor expression display behaviors related to schizophrenia. Cell. 1999;98:427–36.
Newcomer JW, Krystal JH. NMDA receptor regulation of memory and behavior in humans. Hippocampus. 2001;11:529–542.
Ross CA, Margolis RL, Reading SA, Pletnikov M, Coyle JT. Neurobiology of schizophrenia. Neuron. 2006;52:139–153.
Rung JP, Carlsson A, Rydén Markinhuhta K, Carlsson ML. (+)-MK-801 induced social withdrawal in rats; a model for negative symptoms of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:827–832.
Seo TB, Cho HS, Shin MS, Kim CJ, Ji ES, Baek SS. Treadmill exercise improves behavioral outcomes and spatial learning memory through up-regulation of reelin signaling pathway in autistic rats. J Exerc Rehabil. 2013;9:220–229.
Tapia-Arancibia L, Rage F, Givalois L, Arancibia S. Physiology of BDNF: focus on hypothalamic function. Front Neuroendocrinol. 2004;25:77–107.
van Praag H. Exercise and the brain: something to chew on. Trends Neurosci. 2009;32:283–290.
Vaynman S, Ying Z, Gomez-Pinilla F. Interplay between brain-derived neurotrophic factor and signal transduction modulators in the regulation of the effects of exercise on synaptic-plasticity. Neuroscience. 2003;122:647–657.
Weuve J, Kang JH, Manson JE, Breteler MM, Ware JH, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292:1454–1461.
Yoon MC, Shin MS, Kim TS, Kim BK, Ko IG, Sung YH, Kim SE, Lee HH, Kim YP, Kim CJ. Treadmill exercise suppresses nigrostriatal dopaminergic neuronal loss in 6-hydroxydopamine-induced Parkinson’s rats. Neurosci Lett. 2007;423:12–17.
Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550.
Fig. 1.Effect of treadmill exercise on the number of NMDA-receptor-positive cells in the hippocampal dentate gyrus. Upper: Photomicrographs of NMDA-receptor-positive cells in the prefrontal cortex. The scale bar represents 100 μm. Lower: Number of NMDA-receptor-positive cells in each group. (A) Control group, (B) MK-801 injection group, (C) MK-801 injection and treadmill exercise group. The data are represented as mean± SEM. *Represents P< 0.05 compared to the control group. *#Represents P< 0.05 compared to the MK-801 injection group. 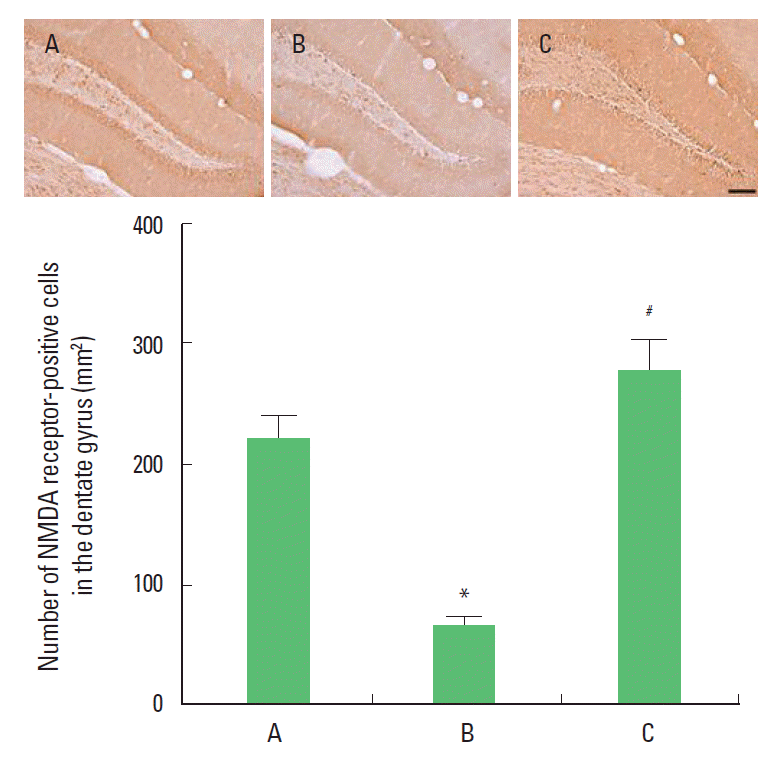
Fig. 2.Effect of treadmill exercise on the number of NMDA-receptor-positive cells in the prefrontal cortex. Upper: Photomicrographs of NMDA-receptor-positive cells in the prefrontal cortex. The scale bar represents 100 μm. Lower: Number of NMDA-receptor-positive cells in each group. (A) Control group, (B) MK-801 injection group, (C) MK-801 injection and treadmill exercise group. The data are represented as mean±SEM. *Represents P< 0.05 compared to the control group. #*Represents P< 0.05 compared to the MK-801 injection group. 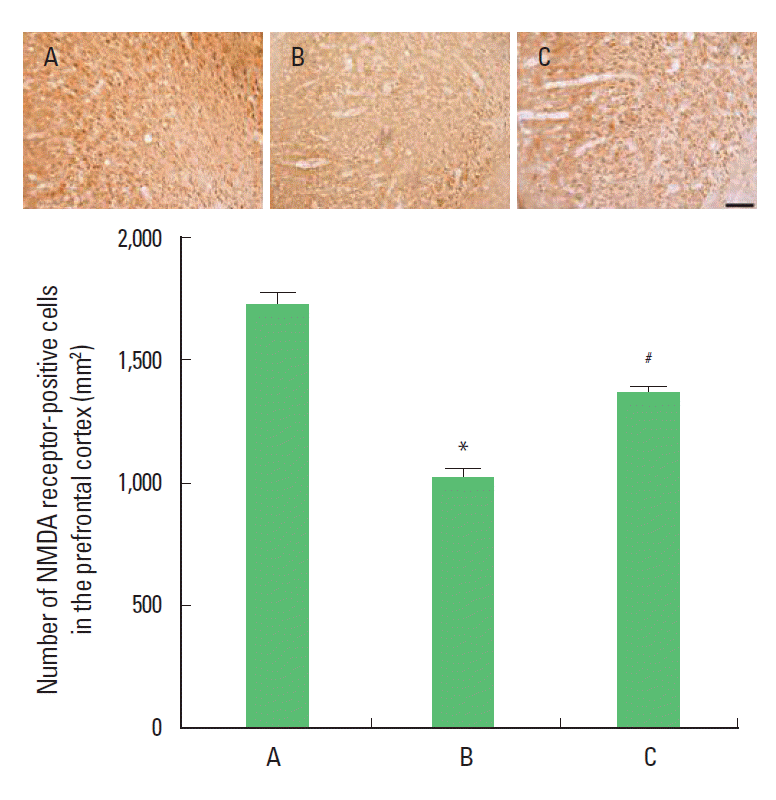
Fig. 3.Effect of treadmill exercise on the TH expression in the striatum. Upper: Photomicrographs of TH expression in the striatum. The scale bar represents 400 μm. Lower: TH expression in each group. (A) Control group, (B) MK-801 injection group, and (C) MK-801 injection and treadmill exercise group. The data are represented as mean±SEM. *Represents P< 0.05 compared to the control group. #*Represents P< 0.05 compared to the MK-801 injection group. 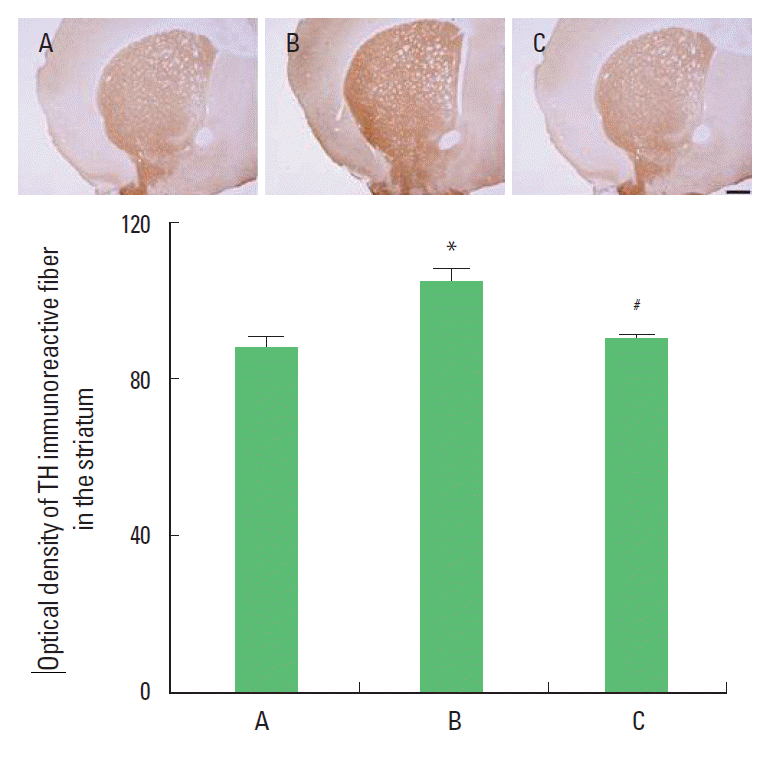
Fig. 4.Effect of treadmill exercise on the expression of BDNF in the hippocampus. Upper: Photomicrographs BDNF expression in the hippocampus. Lower: Expression of BDNF density in each group. (A) Control group, (B) MK-801 injection group, (C) MK-801 injection and treadmill exercise group. The data are represented as mean±SEM. *Represents P< 0.05 compared to the control group. #*Represents P< 0.05 compared to the MK-801 injection group. 
|
|
|||||||||||||||||||||||||||||||||||||||