Endoscopic resection of early gastric cancer
Article information
Abstract
Endoscopic resection (ER) is an effective treatment for early gastric cancer (EGC) without metastases. Existing endoscopic mucosal resection (EMR) is easy to perform, has few complications, and can be applied when the lesion size is small. However, en bloc and complete resection rates vary depending on the size and severity of the lesion. EMR using the cap-mounted panendoscopic method and EMR after circumferential preamputation of the lesion are useful in the treatment of EGC. However, completely oversized lesions (≥2 cm) and lesions associated with ulcers or submucosal fibrosis are more likely to fail resection. Endoscopic submucosal dissection has been widely used to resect tumors larger than 2 cm in diameter and has a higher acceptable complication rate and en bloc and complete resection rates than EMR. ER for EGC is superior to surgical resection in terms of improving patient quality of life. Additionally, compared to surgery, emergency rooms have a lower rate of treatment-related complications, shorter hospital stays, and lower costs. Accordingly, the indications for ER are expanding in the field of therapeutic endoscopy. Long-term outcomes regarding recurrence are excellent in both absolute and extended criteria for ER in EGC. Close surveillance should be performed after ER to detect early metachronous gastric cancer and precancerous lesions that can be treated with ER. Follow-up gastroscopy and abdominopelvic computed tomography scans every 6 to 12 months are recommended for patients who undergo curative ER for EGC on absolute or extended criteria.
INTRODUCTION
Gastric cancer was the fifth most common malignancy in the world in 2020 and the fourth leading cause of cancer death (Sung et al., 2021). There is significant geographic variation in gastric cancer incidence worldwide. In 2020, gastric cancer incidence was highest in Eastern Asia (22.4 per 100,000 people), followed by Central and Eastern Europe (11.3 per 100,000 people), South America, Polynesia, and Western Asia (approximately 8.6 per 100,000 people). Various factors, including environmental and lifestyle factors, are very important in the development of stomach cancer. In 1994, the International Agency for Research on Cancer classified Helicobacter pylori infection as a risk factor for gastric cancer. A correlation between salt intake (high salt, smoked foods, salted fish or meat) and gastric cancer risk has been shown in several epidemiological studies (Fang et al., 2015). Additionally, the American Cancer Society stated in 2021 that smoked foods and pickled vegetables represent a risk factor for stomach cancer. The risk of stomach cancer is significantly increased by smoking (40% in smokers and 82% in heavy smokers) and alcohol consumption (IARC Working Group on the Evaluation of Carcinogenic Risks to Humans, 2004).
Gastric cancer survival rates vary worldwide. Globally, the overall 5-year relative survival rate for gastric cancer is approximately 20%–30% in most regions except Japan and Korea. However, the 5-year survival rate is 67% in Korea and 69% in Japan (Kim et al., 2016; Siegel et al., 2014). These differences are partly explained by the early detection of gastric cancer through screening programs implemented in East Asia (Brenner, 2002). The proportion of early gastric cancer (EGC) among all gastric cancers is steadily increasing due to the increase in gastric cancer screening endoscopy, especially during the National Cancer Screening Program in Korea and Japan (Hamashima and Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines, 2018; Jun et al., 2017). After early detection of EGC, effective treatments for EGC, such as endoscopic resection (ER) and surgical gastrectomy, have contributed to improving the overall 5-year survival rate. Therefore, early detection and appropriate management of EGC and gastric precancerous lesions are important to reduce gastric cancer-related mortality.
EGC is defined as gastric cancer confined to the mucosa or submucosa, regardless of regional lymph nodes (Sano et al., 1992). Gastric dysplasia is considered a precancerous lesion (Dixon, 2002). The risk of carcinoma generally increases with the histological grade of dysplasia (low to high grade). According to the revised Vienna classification (Stolte, 2003), gastric low-grade dysplasia (LGD) is classified as category 3, and ER or regular follow-up is recommended. Category 4 is further classified into category 4.1, defined as high-grade dysplasia (HGD); 4.2, non-invasive carcinoma (carcinoma in situ); 4.3, suspicion of invasive carcinoma based on the degree of structural or cytological atypia of the neoplastic gland; and 4.4, intramucosal carcinoma. It is strongly recommended that gastric HGD with a high predictive potential for carcinoma should be treated with ER or surgical resection.
ER is recognized as a minimally invasive treatment strategy for precancerous gastrointestinal lesions, superficial esophageal cancer, EGC, and early colorectal cancer. Previous studies have shown that the clinical outcomes of ER for EGC are favorable compared to surgical resection (Choi et al., 2015; Pyo et al., 2016). ER for EGC is superior to surgical resection in terms of improving patient quality of life. Moreover, compared to surgery, ER has a lower rate of treatment-related complications (Hahn et al., 2018), shorter hospitalization period, and lower cost (Choi et al., 2015). As a result, the paradigm from surgical resection to ER as a treatment modality for selected EGCs and precancerous gastric lesions is accelerating. This review article will summarize the methods, indications, long-term outcomes, and post-ER management of ER as a treatment for EGC and precancerous gastric lesions.
ENDOSCOPIC RESECTION METHOD
Endoscopic mucosal resection (EMR) and endoscopic submucosal dissection (ESD) are representative endoscopic treatments for EGC and precancerous gastric lesions. EMR was introduced in Japan in 1984 as a procedure in which saline is injected submucosally into the lesion and then the lesion is excised using a snare. Existing EMR is easy to perform, has few complications, and can be applied when the lesion size is small. However, en bloc and complete resection rates vary depending on the size and severity of the lesion. When using conventional EMR methods for lesions less than 1 cm in size, the complete resection rate was approximately 60%, whereas for lesions larger than 2 cm, the complete resection rate was low (20%–30%) (Noda et al., 1997). Cap-mounted panendoscopic EMR, a type of EMR, was applied to treat EGC in 1992 (Inoue et al., 1993). This technique uses a clear plastic cap attached to the end of an endoscope. A snare is pre-looped inside the groove inside the distal end of the cap, allowing the operator to cut the lesion that has been sucked into the cap. EMR after circumferential precutting of the lesion is another modified EMR method. The procedure for performing EMR after precutting the lesion circumferentially is described below. First, the endoscopist uses a brief electrocautery or argon plasma coagulation to make a circumferential mark on the outside of the lesion. Second, a needle is used to cut the lesion outside the mark. After injecting submucosal saline into the lesion, a knife or snare tip is used. Third, the operator uses a snare to excise the lesion. EMR using the cap-mounted panendoscopic method and EMR after circumferential preamputation of the lesion are useful in the treatment of EGC. However, completely oversized lesions (≥2 cm) or lesions associated with ulcers or submucosal fibrosis are more likely to fail resection.
ESD was first introduced in 1999 (Gotoda et al., 1999) and has been used to remove EGCs and precancerous gastric lesions (Figs. 1 and 2). The procedure is as follows (1) external circumferential marking of the lesion, (2) submucosal saline injection into the lesion, (3) circumferential external marking using a knife, and (4) submucosal dissection using a knife. Using this technique, the submucosa is incised with an endoscopic knife. A variety of knives were used to incise the submucosa parallel to the appropriate muscle layer. ESD can be used to remove tumors exceeding 2 cm in diameter and lesions associated with ulcers or submucosal fibrosis.
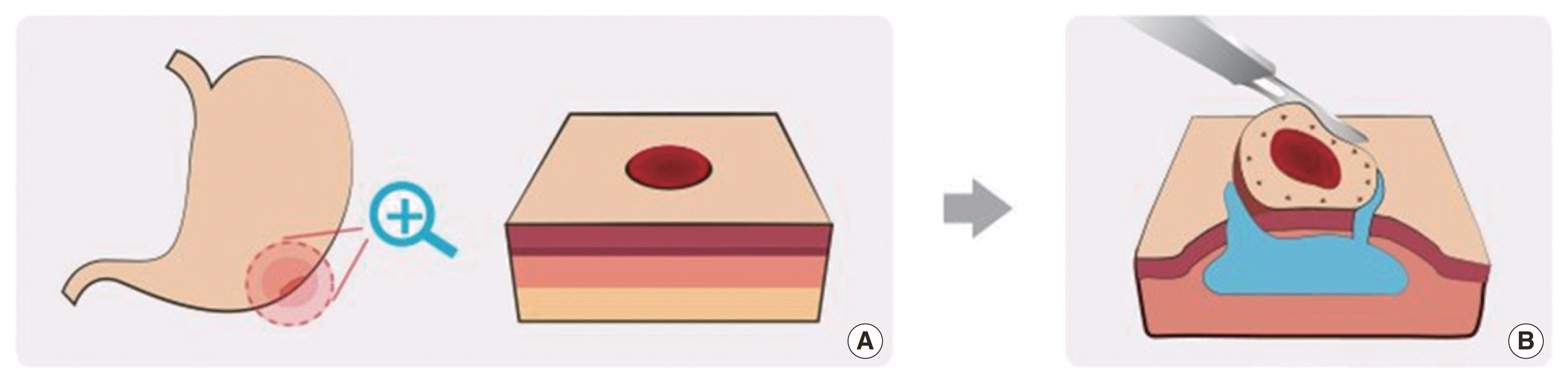
Endoscopic submucosal dissection (ESD). (A) Detection of early gastric cancer using gastroscopy. (B) The ESD procedure is as follows: (1) circumferential marking outside the lesion, (2) submucosal saline injection for the lesion, (3) circumferential precutting outside marking using knives, (4) submucosal dissection using knives.
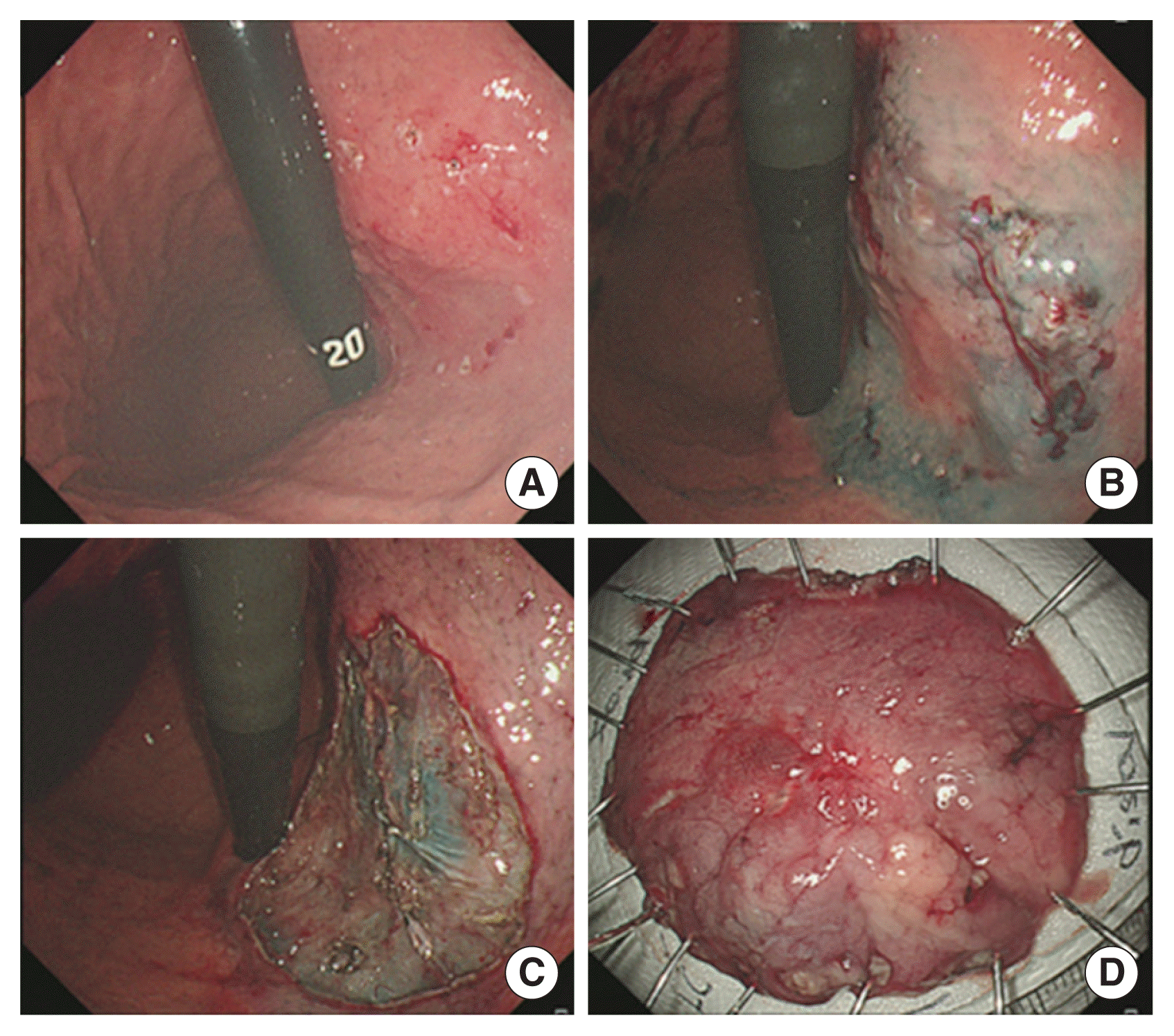
Endoscopic findings of endoscopic submucosal dissection. (A) Marking for early gastric cancer (EGC) at posterior wall of upper body. (B) Submucosal saline injection for EGC. (C) Circumferential precutting outside marking and submucosal dissection using knives for EGC. (D) Pinning of resected specimen.
INDICATIONS FOR ENDOSCOPIC RESECTION
According to the revised Vienna classification (Stolte, 2003), ER or regular follow-up is recommended for gastric LGD. Gastric HGD, which has a high predictive value for carcinoma, is recommended to be treated with ER or surgical resection. In 2021, the Korean ESD Study Group reported that Helicobacter pylori infection, smoking history, tumor location in the lower third of the stomach, tumor size >10 mm, pitting lesions, and ulcers were significant predictive factors for prestage diagnosis of EGC in LGD (Jeon et al., 2021). They also reported that previous history of gastric cancer, Helicobacter pylori infection, smoking history, tumor location in the lower third, tumor size >10 mm, pitting lesions, and ulcers were significant predictive factors for a prestage diagnosis of EGC in HGD after ER. Therefore, precise ER procedures, such as ESD, are recommended for LGD and HGD where factors predict pathological elevation to EGC.
The absolute indications for ER as a treatment for EGC are as follows (Japanese Gastric Cancer Association, 2011): (1) highly or moderately differentiated adenocarcinoma, (2) diameter less than 2 cm, (3) no ulcer within the tumor, (4) Intramucosal cancer. This indication was established based on the technical limitations of EMR. According to a previous study of the prevalence of LNM in 5,265 patients who underwent gastrectomy with careful lymph node dissection over a 30-year period, the risk of LNM was 0% if the mucosal differentiated lesion was 2 cm or less without ulcers (Gotoda et al., 2000).
Compared with EMR, ESD allows for en bloc resection of large or ulcerated lesions. Additionally, using ESD, lesions can be collectively excised down to the submucosal layer. Gotoda et al. (2000) found that, regardless of size, mucosal differentiated lesions without ulcers, mucosal differentiated ulcerated lesions less than 3 cm, poorly differentiated mucosal lesions less than 2 cm, and lesions less than 3 cm with some submucosal invasion (<500 μm) with no lymphatic invasion, the risk of LNM was 0%. Therefore, expanded criteria for ER of EGC have been proposed as follows: (1) intramucosal differentiated adenocarcinoma without ulcers, size greater than 2 cm, (2) intramucosal differentiated adenocarcinoma with ulcers, size ≤3 cm, (3) submucosal invasion less than 500 μm, differentiated adenocarcinoma, size ≤3 cm, and (4) intramucosal undifferentiated adenocarcinoma without ulcers, size ≤2 cm (Table 1).
LONG-TERM OUTCOMES AFTER ENDOSCOPIC RESECTION FOR EARLY GASTRIC CANCERS
The 5-year overall survival (OS) of ER patients was not significantly different from the 5-year OS of patients who received ER (93.6%–96.4%) and surgery (94.2%–97.2%) (Choi et al., 2015; Hahn et al., 2018). The 10-year OS rate was similar between ER (81.9%) and surgery (84.9%) (P=0.14) (Choi et al., 2011). The 5-year OS rate for patients meeting expanded criteria who received ER for EGC treatment was 94.8%–99.5% (Shichijo et al., 2021), which was similar to the rate for patients meeting absolute indications for receiving ER. Nonrandomized studies have compared the long-term outcomes of ER and surgery in patients meeting expanded criteria (Choi et al., 2015; Lee et al., 2018; Pyo et al., 2016). The 5-year OS rate for patients meeting expanded criteria who received ER was 93.6%–98.1%, compared with 85.8%–97.3% for patients meeting expanded criteria who underwent surgery.
A recent multicenter prospective cohort study of ER in EGC demonstrated an overall 5-year OS of 89.0% (95% confidence interval, 88.3%–89.6%) (Suzuki et al., 2023). In multivariate analysis, no significant differences were observed when the hazard ratios for all-cause mortality for expanded criteria were compared with absolute indications. This study also compared 5-year OS with expected OS calculated for patients with surgically resected EGC. ER was considered effective if the lower bound of the 95% confidence interval for 5-year OS exceeded the expected 5-year OS minus a 5% margin (threshold 5-year OS). The lower bound of the 95% confidence interval for 5-year OS exceeded the 5-year OS threshold in both absolute and extended categories. These results imply that ER can be recommended as standard treatment for patients with EGC who meet both absolute and extended criteria.
The 5-year cumulative metachronous gastric cancer recurrence rate was significantly higher after ER (5.8%–10.9%) than the postoperative recurrence rate (0.9%–1.1%) (Choi et al., 2011; Choi et al., 2015; Hahn et al., 2018). This is because the stomach is preserved intact after ER compared to surgical resection. Therefore, close surveillance after ER should be performed to detect early metachronous gastric cancer that can be treated with ER. According to a study on the long-term effectiveness of Helicobacter pylori eradication therapy for preventing metachronous gastric cancer after ER (Choi et al., 2018), a total of 396 patients underwent ER for EGC or HGD and received Helicobacter pylori eradication therapy with antibiotics or placebo was included (194 in the treatment group, 202 in the placebo group). During a median follow-up of 5.9 years, 14 patients (7.2%) in the treatment group and 27 patients (13.4%) in the placebo group developed metachronous gastric cancer (hazard ratio in the treatment group, 0.50; 95% confidence interval, 0.26–0.94; P=0.03). Therefore, to prevent the development of metachronous gastric cancer in Helicobacter pylori patients, Helicobacter pylori eradication therapy should be performed after ER.
CARE AFTER ENDOSCOPOIC RESECTION
Management in patients receiving ER is shown in Fig. 3. If a lesion is resected en bloc, lymphovascular invasion is negative, and surgical margins are negative, it is considered a curable lesion (Ono et al., 2016). Follow-up gastroscopy every 6 to 12 months is recommended for patients who have undergone curative ER for EGC on absolute or extended criteria. Patients who have previously tested negative for Helicobacter pylori infection are recommended to be tested for Helicobacter pylori infection every 12 months. If Helicobacter pylori is confirmed to be present in the stomach, Helicobacter pylori eradication therapy is required. Additionally, it is recommended to perform regular abdominopelvic computed tomography at 6- to 12-month intervals after curative ER of EGC to detect extragastric recurrence.
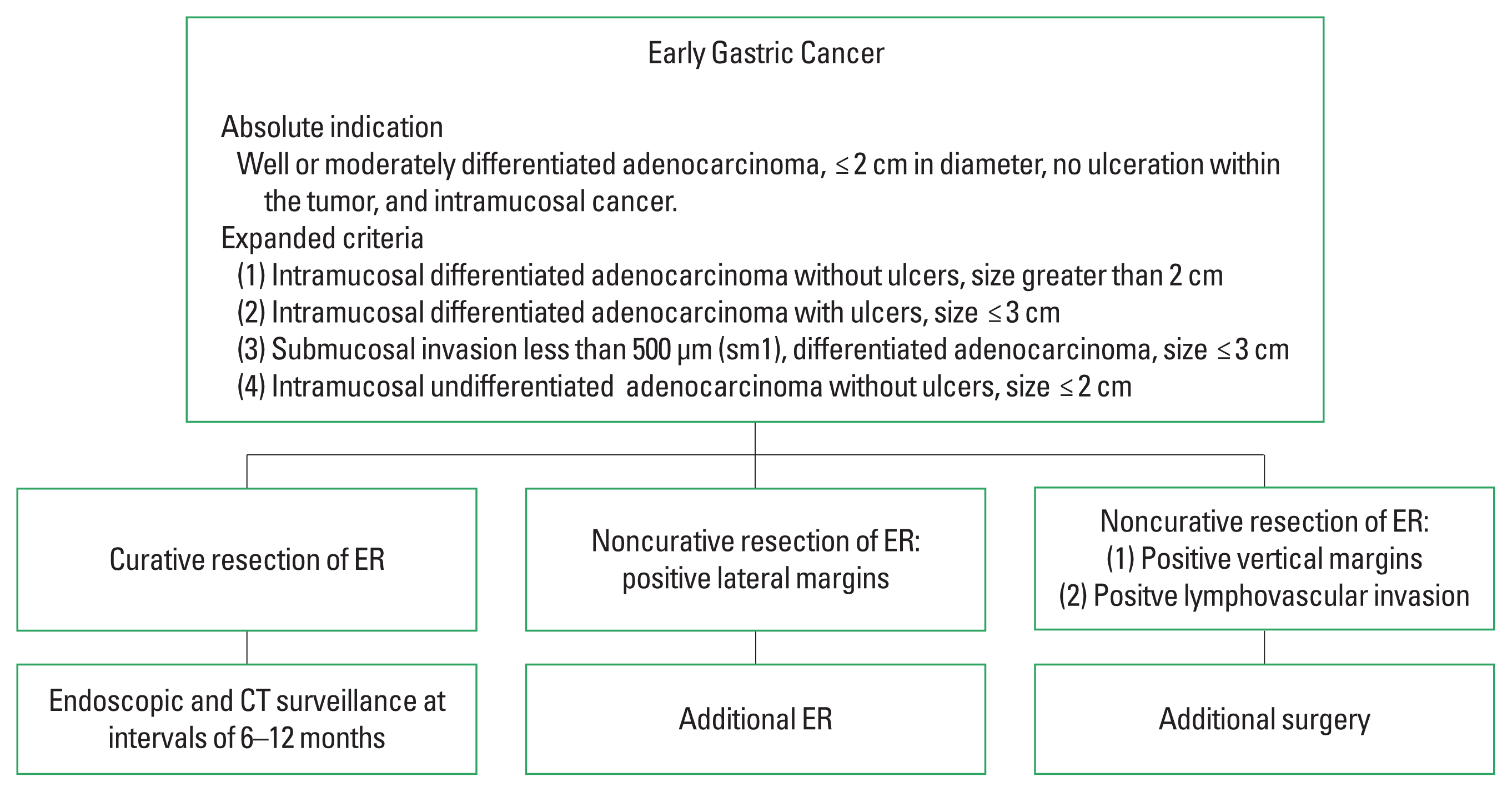
Proposal for the management in patients undergoing endoscopic resection (ER) for early gastric cancer (EGC) based on the absolute or expanded criteria. CT, computed tomography.
In the field of therapeutic endoscopy, the indications for ER have expanded. As a result, noncurative resection may occur unintentionally by the operator. The incidence of noncurative resection after ER of EGC has been reported to be approximately 11.9%–18.5% (Oda et al., 2008). Previous study has analyzed risk factors affecting noncurative resection of ER of EGC, including large tumor size, tumor location, presence of ulcers, and undifferentiated tumor (Hatta et al., 2020). The standard treatment for lesions presenting with benign lymphovascular infiltration or positive vertical margins diagnosed after ER is additional surgery (Ono et al., 2016). However, if histopathological evaluation of endoscopically resected EGC specimens shows benign involvement of the horizontal resection margin border, additional ER rather than additional surgery may be indicated (Hatta et al., 2017).
ACKNOWLEDGMENTS
This study was supported by a grant from the National Research Foundation of Korea (NRF-2022R1F1A1073313).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.

