Oxidative stress associated with impaired autonomic control and severity of lung function in chronic obstructive pulmonary disease patients
Article information
Abstract
Oxidative stress has been suggested to play a role in the pathogenesis of chronic obstructive pulmonary disease (COPD). This study aimed to investigate a link between malondialdehyde (MDA) levels, pulmonary function, and cardiac autonomic control in patients with COPD. Plasma levels of MDA, heart rate variability, and pulmonary function were measured in 50 clinically stable COPD patients and 50 normal male controls. COPD patients exhibited lower means of the standard deviations of all normal to normal (NN) intervals (SDNN), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD), and high frequency (HF). Nevertheless, they presented greater low frequency (LF) and low frequency/high frequency ratio (LF/HF ratio) in supine and head-up tilt positions than controls (P<0.001). More-over, a negative correlation between MDA levels with SDNN (P<0.001) and a positive correlation with LF (P<0.01) and LF/HF ratio (P<0.05) were observed in both positions. In COPD patients, plasma MDA levels were 2.3 times greater than controls (4.33±2.03 μM vs. 1.89±0.39 μM, P<0.001), and they were inversely correlated with forced vital capacity, forced expiratory volume in 1 sec, midexpiratory flow, and peak expiratory flow (P<0.001). Our findings suggest a potential role for oxidative stress in impaired cardiac autonomic control and clinical relevance of plasma MDA levels as a predictor of severity of COPD in COPD patients.
INTRODUCTION
The main reason for chronic obstructive pulmonary disease (COPD) is smoking, with smoking duration and packs per year were related with the severity of COPD (Toghyani and Sadeghi, 2022). Cardiovascular disease (CVD) is common in individuals with COPD and is likely the most common comorbidity of COPD. Its presence is related to an increased risk of hospitalization, longer length of stay, and all-cause mortality associated with CVD (Morgan et al., 2018). Interestingly, previous meta-analysis of observational studies supports a two-fold significant increase in the odds of having any CVD in those with COPD relative to COPD-free patients (odds ratio [OR], 2.46; 96% confidence interval [CI], 2.02–3.00), and ORs in the range 2–5 for arrhythmias, heart failure, ischemic heart disease, and atherosclerosis of peripheral arteries (Chen et al., 2015).
The autonomic nervous system is involved in sympathetic and parasympathetic controls in addition to regulation of the internal physiology of the body including blood pressure and heart rate (HR). HR variability (HRV) in a cardiac autonomic nervous marker is defined as one of the factors contributing to pathophysiology in COPD. The literature suggests that patients with COPD have elevated resting HR, reduced baroreflex sensitivity, altered autonomic nervous control involvement, decreased parasympathetic activity, and/or increased sympathetic activity relative to healthy subjects (Huertas and Palange, 2011; Mohammed et al., 2015; Serrao et al., 2020; Tseng et al., 2018), all of which may be linked to morbidity and mortality (Stein et al., 1998). In addition, depression of cardio autonomic control has been reported to have a positive association with the severity stage of COPD (Haider et al., 2009). In recent decades, accumulated evidence has clearly shown a central role for increasing sympathetic nervous activity relative to an induced inflammatory process (Pongratz and Straub, 2014) which leads to increased oxidative stress in COPD patients (Wiegman et al., 2015; Wiegman et al., 2020).
Oxidative stress characterized by an imbalance between generation of reactive oxygen species and the capacity of the intrinsic antioxidant defense system, subsequently induces DNA damage, lipid peroxidation, impedes mitochondrial dynamics, interrupts mitophagy, and protein modification (Pizzino et al., 2017; Scandalios, 2005; Yoo et al., 2018). Prolonged imbalance and chronic oxidative stress from smoking causes a direct injury to the respiratory tract and is responsible for the pathogenesis of COPD in smokers (Barnes, 2022; Cavalcante and de Bruin, 2009). Lipid peroxidation plays a role in COPD pathogenesis (Barnes, 2020). This causes pulmonary damage or elicits diverse cellular responses through the formation of species with secondary metabolic interactions (Bajpai et al., 2017). Lipid peroxidation by either nonenzymatic or enzymatic mechanisms is a direct outcome of oxidative stress resulting in oxidative damage (Niki, 2009). The important biomarker is frequently used to characterize the state of systemic lipid peroxidation is malondialdehyde (MDA), which has been determined in various diseases such as coronary heart disease (Bastani et al., 2019), hypertension (Verma et al., 2019), diabetes mellitus (Mahreen et al., 2010) as well as COPD (Paliogiannis et al., 2018). MDA, a product of lipid peroxidation, is elevated in patients with COPD compared to healthy subjects (Arja et al., 2013; Paliogiannis et al., 2018), and during exacerbation of COPD (Dekhuijzen et al., 1996). Some previous studies reported that plasma MDA level was correlated inversely with the degree of small airway obstruction (Petruzzelli et al., 1990) and pulmonary function (Arja et al., 2013), while there was one a study found no significant correlation between forced expiratory volume in 1 sec (FEV1), FEV1/forced vital capacity (FVC), and the levels of plasma MDA in patients with COPD (Altuntas et al., 2003).
Pulmonary rehabilitation is an overall multidisciplinary intervention aimed at reducing symptoms and improving functional activity and quality of life. These include medical investigations, physical and medical procedures, and reassessments. In addition, risk factors for CVD, severity of lung function, and oxidative stress were performed to assess the prognosis in patients with COPD (Ahn et al., 2015; Arja et al., 2013). Although the correlation between oxidative stress markers and lung function have been reported, the results of previous studies have shown inconsistent results. Furthermore, there have been no studies on the relationship between oxidative stress markers and HRV in COPD patients at risk factor of CVD. Therefore, the present study aimed to investigate a link between MDA and pulmonary function, and cardiac autonomic control in patients with COPD.
MATERIALS AND METHODS
Design and population
The number of COPD patients was calculated according to a previous study by Camillo et al. (2008). They performed HRV analysis to evaluate the presence of autonomic dysfunction in patients with COPD. The sample size was calculated by using the formula N=Z21–α/2 σ2/d2, where: N=number of subjects, Z21–α/2=CI (95%), σ=standard deviation (SD) (18), d=precision (5). In the present study, the number of COPD patients was 50. The experimental design of this study is shown in Fig. 1. A total of 200 COPD patients and 65 healthy controls were evaluated for eligibility. As a result of inclusion and exclusion criteria, 150 patients with COPD and 15 healthy controls that did not meet inclusion criteria were excluded. Thus, 50 COPD patients and 50 healthy controls were divided into the COPD and control groups. Fifty clinically stable patients with mild to very severe COPD with a smoking history of 40.2±23.0 packs per year, and 50 nonsmoking with no history of disease male controls were investigated. Male control subjects were recruited from primary health care centres in Khon Kaen province, Thailand. Patients with COPD were enrolled from the COPD clinic at Srinagarind Hospital. Posters with the study details were posted by staff at the primary health care centres and Srinagarind Hospital, Khon Kaen University, Thailand. Participants who had attended primary health care services or the COPD clinic at Srinagarind Hospital and were interested in taking part in the study contacted a staff member or research assistant via telephone. Informed consent was obtained from each subject, including purpose, benefits, and possible risks associated with the experiments in accordance with the Khon Kaen University Ethics Committee for Human Research (approval numbers HE571287). The Thai Clinical Trials Registry (TCTR) identification number is TCTR20160607001.
The diagnosis of COPD and its severity were determined using the global initiative for chronic obstructive lung disease (GOLD) 2018 guidelines (Mirza et al., 2018). Patients treated with short-acting beta-2 agonists (SABA), long-acting beta-2 agonists (LABA), inhaled corticosteroids (ICS), LABA+ICS, long-acting muscarinic antagonists (LAMA), and methylxanthine were 60%, 18%, 92%, 18%, 6%, and 66% of total COPD patients, respectively. None of the COPD patients had COPD exacerbation within the 3 months prior to the start of the study. Subjects with a history of cardiac arrhythmias or potential electrocardiograph (ECG) alterations, history consistent with heart disease, diabetes mellitus, hypertension or other concomitant respiratory diseases, central or peripheral nervous system diseases, and electrolyte imbalance were excluded from the study.
HRV measurement
HRV was measured via autoregressive power spectral analysis during 10 min of R-R interval via a 3-lead ECG (PowerLab 26T ADInstruments, Sydney, New South Wales, Australia). Both supine and head-up tilt readings were taken for at least 5 min in each position using a tiltable bed. After 10 min of rest in the supine position, the ECG was recorded for at least 5 min in the same position and then the tiltable bed was adjusted to 70 degrees. During the changing of position, the experiment was discontinued if subjects presented adverse reactions such as dizziness or malaise. The HRV analysis was performed in time and frequency domains. For analysis in the time domain, the mean of the SDs of all normal to normal (NN) intervals (SDNN) was employed as a measure of the sum of sympathetic and parasympathetic activities. The square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD) was used as a measure of parasympathetic activity. For analysis in the frequency domain, low frequency spectral power (LF power, the density of the beat-to-beat oscillation in the NN interval of HRV in the low frequency band; LF=0.04–0.15 Hz) due to sympathetic activity and to a lesser extent vagal activity on the heart was used. High frequency spectral power (HF power, the density of the beat-to-beat oscillation in the NN interval of HRV in the high frequency band; HF= 0.15–0.4 Hz) is an indicator of vagal activity on the heart. The low frequency/high frequency ratio (LF/HF ratio) has been described as an index of sympathetic to parasympathetic balance or sympathovagal balance. Both the LF and HF powers were expressed in normalized units (n.u.) being measured as percentages of LF or HF in the total power spectrum (n.u. Percent=[power of LF or HF]/[total power–very low frequency power]).
Pulmonary function test
A Vitalograph Pneumotrac (Vitalograph, Ennis, Co Clare, Ireland) was utilized to measure pulmonary function. They were read from the best of three recordings performed in the standing position with a nose clip in place, according to the American Thoracic Society and European Respiratory Society Standardization of Lung Function Testing. All values were expressed as percentages of the predicted normal values.
Oxidative stress marker
Blood was collected in an ethylenediaminetetraacetic acid tube and centrifuged at 3,500 rpm, for 10 min, at 4°C. Plasma was collected to determine the level of MDA by measuring thiobarbituric acid reactive substances as previously mentioned (Somparn et al., 2007).
Statistical analysis
Statistical analyses were performed using Stata 12.0 (StataCorp LLC, College Station, TX, USA). Data were reported as mean±SD. The Shapiro–Wilk test was utilized to test the normal distribution of the data. A Student t-test was used to determine between-group differences when data were normally distributed in parameters including age, diastolic blood pressure, HR, FEV1, FVC, FEV1/FVC ratio. The Wilcoxon rank-sum test was utilized in some parameters when data deviated from normality including body mass index, systolic blood pressure, MDA level, and HRV parameters. The correlation between pulmonary function and HRV or MDA levels was assessed using Pearson’s correlation analysis. The threshold for statistical significance was set at P<0.05 for all analyses.
RESULTS
Demographic parameters of all participants
The average age was 64.06±8.43 years in the control group and 66.47±9.47 years in the COPD group. Table 1 demonstrates that the body mass index of COPD patients was significantly lower compared to those of controls (P<0.001). Moreover, the percentages predicted amid pulmonary function parameters consisting of FEV1, FVC, and FEV1/FVC ratio were significantly decreased in COPD patients (P<0.001). HR was significantly greater in COPD patients compared to that of control subjects (80.72±12.37 beats/min vs. 74.34±9.73 beats/min, P<0.05). systolic blood pressure and diastolic blood pressure in COPD patients were comparable to controls and fell within normal ranges. Patient with COPD exhibited a smoking history 40.20±23.0 packs per year, and medication usage as follows: ICS 92%, LABA 18%, LABA+ ICS 18%, LAMA 6%, methylxanthine 66%, and SABA 60%, respectively (Table 1).
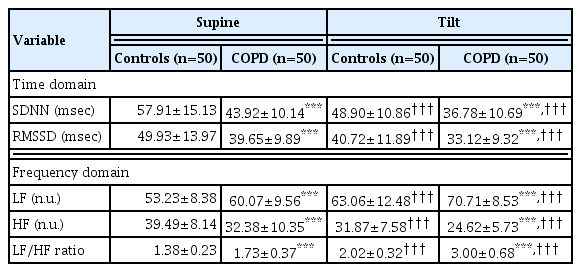
HRV parameters in supine and tilt positions
HRV is composed of two domains: first, time domain consists of SDNN, and RMSSD which indicates HRV to quantify the amount of variability in measurements of the inter-beat interval which is the period between successive heartbeats. Secondly, the frequency domains include LF-sympathetic activity, HF-parasympathetic activity, and the LF/HF ratio-estimate for the relationship between the activity of the sympathetic and parasympathetic nervous systems. An overview of HRV measurements in COPD patients and controls is given in Table 2. In relation to the supine and tilt-period positions, they demonstrated significant differences in all HRV variables - both in the time domain and in the frequency domain. In the COPD group, significant decreases in SDNN, RMSSD, and HF, yet increases in LF and LF/HF ratio in both positions compared to normal control were observed (P<0.001) (Table 2, Fig. 2). These data suggest that patients with COPD had greater sympathetic and lower parasympathetic activity than the normal control.

Heart rate variability (HRV) parameters, the standard deviations of all normal to normal (NN) intervals (SDNN) (A), the square root of the mean of the sum of the squares of differences between adjacent NN intervals (RMSSD) (B), low frequency (LF) (C), high frequency (HF) (D), and low frequency/high frequency ratio (LF/HF ratio) (E) in supine and head-up tilt position in control (n=50) and chronic obstructive pulmonary disease (COPD) patients (n=50). ***P<0.001 vs. normal controls subjects.
Plasma MDA in all participants
Fig. 3 shows that the plasma MDA concentrations in patients with COPD were 2.3 times greater than in normal patients (P< 0.001). Moreover, MDA levels significantly increased in proportion to the severity of COPD. Plasma MDA levels in each stage of COPD were as follows: GOLD stage I: 2.86±0.22 μM; GOLD stage II: 3.47±0.79 μM; GOLD stage III & IV: 6.52±2.01 μM. Significant variances were revealed between patients with different GOLD stages, and between COPD patients in each GOLD stage versus the control group (Fig. 3).
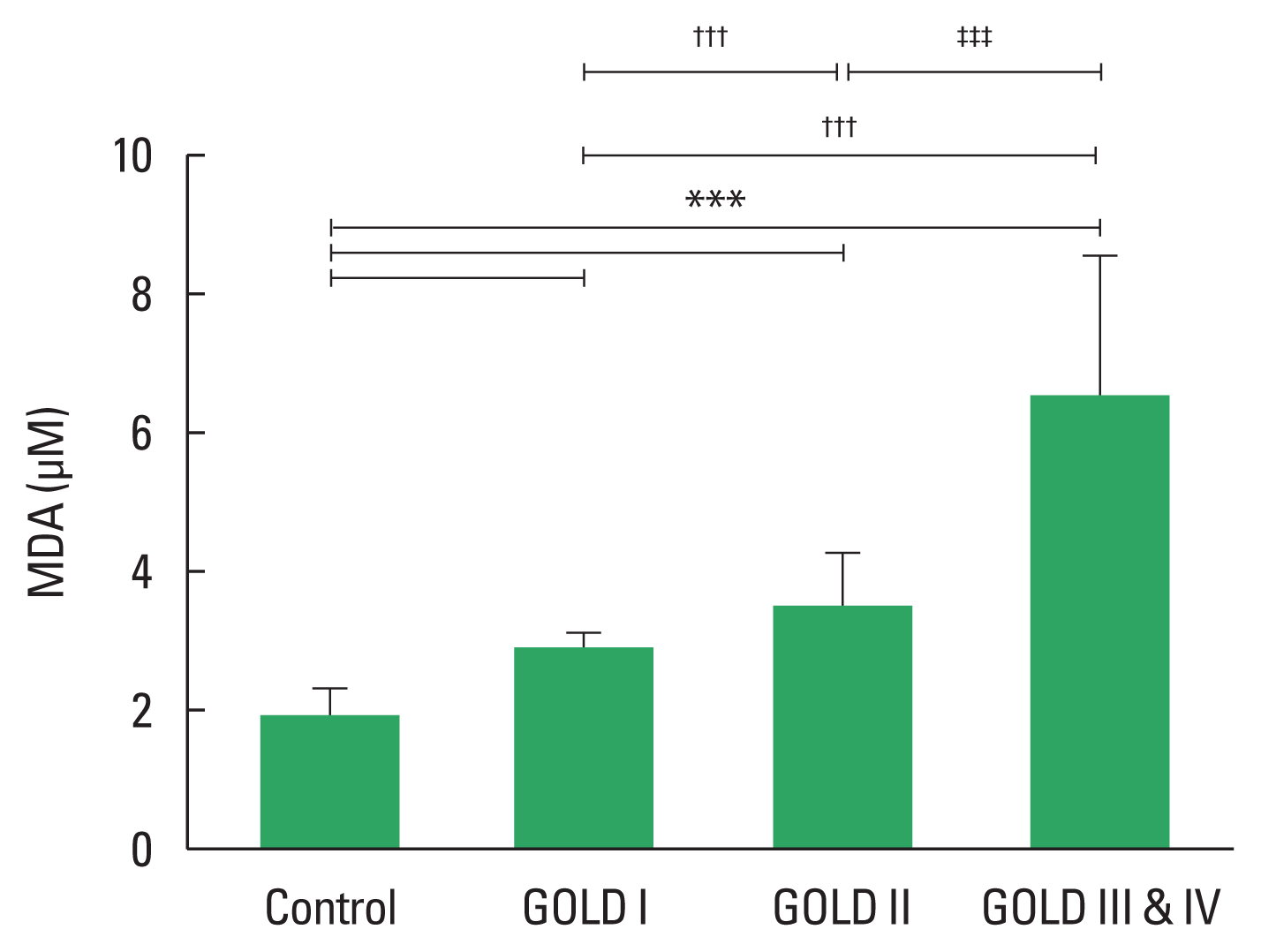
Malondialdehyde (MDA) levels by the global initiative for chronic obstructive lung disease (GOLD) stage of chronic obstructive pulmonary disease (COPD) severity in normal control subject (n=50), GOLD I (n=14), GOLD II (n=19), and GLOD III and V (n=17). ***P<0.001 vs. controls, †††P<0.001 vs. GOLD I, ‡‡‡P<0.001 vs. GOLD II.
Correlation between plasma MDA and pulmonary function in COPD patients
Plasma MDA levels in COPD patients were moderately inversely correlated with pulmonary function as assessed via % predicted FVC (r=−0.505, P<0.001) (Fig. 4A), FEV1 (r=−0.748, P<0.001) (Fig. 4B), midexpiratory flow (MEF) (r=−0.635, P<0.001) (Fig. 4C), and peak expiratory flow (r=−0.518, P<0.001) (Fig. 4D). This implies that elevation of oxidative stress leads to increased severity of lung function in COPD patients.
Correlation between plasma MDA and HRV in COPD patients
The HRV parameters were measured at rest in the supine position. The data found a negative correlation between plasma MDA levels and SDNN (r=−0.822, P<0.001) (Fig. 5A). Instead, plasma MDA levels were positively associated with the LF (r=0.627, P<0.001) (Fig. 5B) and LF/HF ratio (r=0.30, P<0.05) (Fig. 5C). The assessment of cardiac autonomic response HRV in the head-up tilt position was also performed. This was consistent with the supine position. The results showed significant inverse correlation between plasma MDA and SDNN (r=−0.696, P<0.001) (Fig. 5D), positive correlation with the LF (r=0.433, P<0.01) (Fig. 5E) and LF/HF ratio (r=0.309, P<0.05) (Fig. 5F), respectively. These results suggest that the rise in oxidative stress is related to the increase in sympathetic activity in patients with COPD.

The correlation between malondialdehyde (MDA) level and the standard deviations of all normal to normal (NN) intervals (SDNN) (A, D), low frequency (LF) (B, F), and low frequency/high frequency ratio (LF/HF ratio) (C, F) in chronic obstructive pulmonary disease patients in supine (A–C) and head-up tilt (D–F) positions (n=50).
DISCUSSION
The objective of this study was to determine the relationship between oxidative stress markers, pulmonary function, and HRV in COPD patients. Our study demonstrates that plasma MDA levels are related to declines in lung function and autonomic cardiac dysfunction. This finding supports the idea that oxidative stress has a crucial role in the pathophysiology of COPD. Oxidative stress plays an important role in the risk factors amid CVD in patients with COPD (Chen et al., 2015). These results showed significantly increased plasma MDA levels in COPD patients compared to normal controls. This suggests that COPD patients have greater oxidative stress than healthy subjects. Our results agree with previous reports (Arja et al., 2013). The presence of reactive oxygen species in tobacco smoke can damage alveolar epithelial cells by lipid peroxidation of cell membranes and, as a result, increase MDA concentration in plasma. The data of this study also revealed the significant negative correlations between plasma MDA levels and pulmonary function. This can reveal a link between plasma levels of MDA and the severity of the disease. Our observation of this inverse correlation agrees with previous studies that demonstrated the presence of systemic oxidative stress as important in the decline of lung function in COPD (Arja et al., 2013). An increase in oxidizing agents can induce inflammation whereby mediators of inflammation in turn increase the number of oxidizing species, and pulmonary function subsequently destroys it (Drost et al., 2005).
The autonomic nervous system consists of sympathetic and parasympathetic controls and regulation of the internal physiology of the body. The two branches of the autonomous system exert antagonist effects on most bodily functions, contributing to homeostasis in the body, and are responsible for maintaining involuntary vital parameters, including blood pressure, and HR. Autonomic function can be evaluated by monitoring a specific marker of the neurophysiological state of the autonomic system. HRV, which is the variability in time and/or frequency of successive R waves of the heartbeat reflects the integration between the cardiovascular system and the mechanisms it regulates (Shaffer and Ginsberg, 2017). Most recently, autonomic cardiovascular neuropathy has been reported to play a significant role in CVD (Mehra et al., 2022). Although, the evidence in patients with COPD reveals decreased parasympathetic activity and/or increased sympathetic activity compared to healthy subjects, the results were inconsistent (Bartels et al., 2003; Chen et al., 2006; Huertas and Palange, 2011; Mohammed et al., 2015; Serrao et al., 2020; Tseng et al., 2018).
This study showed decreases in SDNN, RMSSD, and HF, yet increases in LF and the ratio of LF/HF in patients with COPD in the resting position when compared to controls. To confirm results from the supine position, we also assessed cardiac autonomic response in the head-up tilt position. This was consistent with the supine position. The decrease in SDNN reflects reduced HRV, whereas decreases in RMSSD and HF reflect the reduced vagal activity. Additionally, the increases in LF and LF/HF ratio are indicative of sympathetic overactivity, and impaired sympathovagal balance, respectively. These findings indicate the presence of autonomic dysfunction in COPD patients and thus greater resting HR than controls. Elevated resting HR may be due to autonomic dysfunction as a result of chronic hypoxia (Warnier et al., 2014). Reduced SDNN and RMSSD found in COPD patients is consistent with a previous study (Volterrani et al., 1994). The increase in sympathetic activity may be due to COPD-related neurohumoral activation, increased release of catecholamines (Andreas et al., 2005), impaired baroreflex sensitivity (Raupach et al., 2008), increased markers of inflammation (Sajadieh et al., 2004), hypoxemia (Chen et al., 2006), and oxidative stress (Van Gestel et al., 2011). It is possible that sympathetic overactivity causes suppression of the sympathoinhibitory cardiovascular reflexes and augmentation of the sympathoexcitatory reflexes (Watson et al., 2006). On the other hand, a previous study reported no change in LF, reduced total HRV, and increased HF in patients with COPD (Volterrani et al., 1994). Chronic hypoxemia in COPD may cause hypoxic damage to the nerves which leads to enhanced cardiac vagal activity and depressed sympathetic activity in COPD patients (Stewart et al., 1991). Previous studies have shown that COPD patients exhibit reductions in both sympathetic and parasympathetic activity as well as demonstrating baroreflex sensitivity damage and reduction of the vagal activity on the sinusal node (Pantoni et al., 2007).
Additionally, previous studies have indicated that oxidative stress plays a critical role in the abnormal sympathoexcitation in several diseases including hypertension and chronic heart failure (Kishi, 2012), though no studies have investigated the relationship between oxidative stress and sympathetic activity in COPD patients. Interestingly, this study showed a negative correlation between plasma MDA and SDNN, LF, and LF/HF ratio in COPD patients. We suggest that oxidative stress may induce sympathetic overactivity in patients with COPD. This results in impairment of the autonomous cardiac system in patients with COPD. Interestingly, it is difficult to predict the lifespan and CVD of patients with COPD. Reliable prognostic markers are valuable and important information for patients, their families, and the physician (Dolan and Varkey, 2005). Therefore, the information from this study could be used as a level of oxidative stress to predict CVD events, pulmonary function severity, and autonomic cardiac dysfunction in patients with COPD. There are some limitations in this study. First, we evaluated only male COPD subjects, which might result in bias. Second, the purpose of this study was not to regulate the use of LABA the day prior to measurements which may influence HRV. Finally, it is possible that other HRV evaluations, such as a 24-hr ambulatory ECG, could be more accurate in measuring HRV.
In conclusion, the present study suggests the presence of sympathovagal imbalance in COPD patients, and measurement of plasma MDA may be useful for assessing the severity of COPD and cardiac autonomic function in COPD patients. Moreover, HRV measurement in COPD patients may present important implications regarding prognosis in CVD amid COPD.
ACKNOWLEDGMENTS
This study was funded by the Thailand Science Research and Innovation funds and the University of Phayao (grant No. FF64-UoE023). The authors would like to thank the COPD patients for participating in the present study.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.