Effects of resistance exercise on adipokine factors and body composition in pre- and postmenopausal women
Article information
Abstract
The objective of the present study was to examine effects of resistance exercise for 12 weeks on adipokine factors and body composition in postmenopausal (POM) women to provide basic data for preventing obesity or metabolic syndrome caused by menopause. Subjects of this study were 35 premenopausal (PRM) and POM women with body fat percentages of 30% or more. They were divided into PRM (n=15) and POM (n=20) groups. All subjects participated in resistance exercise training for 12 weeks. All serum samples were submitted for enzyme-linked immunosorbent assay measurements of adipokine factors. Body weight, muscle mass, body mass index, and waist-to-hip ration showed significant differences between the two groups after training. In contrast, body fat percentage did not differ between the groups, although it was significantly lower in the PRM group after exercise. Physical fitness was significant differences between the two groups after training, including grip strength (left and right), sit and reach, sit-ups, and standing long jump. In addition, grip strength (left), sit-up, and side step tests were significantly increased after exercise in the PRM group. There were the significant differences in interleukin-6 and leptin levels between the two groups after training. Interleukin-6, interleukin-15, and adiponectin levels were significantly higher in both groups after training compared to those before training, although leptin levels were significantly lower after exercise in the PRM group. Regular resistance exercise was found to be effective in decreasing body fat in PRM women, and decreased leptin and increased adiponectin were positively significant in both groups.
INTRODUCTION
Most women in menopausal transition experience hot flashes or regret their lives (Avis et al., 2009; Gold et al., 2006). It has been reported to changes in body composition including obesity and metabolic syndrome (Abdulnour et al., 2012). These changes in body composition are related to levels of physical activity. Many studies have reported that lack of physical activity is linked to obesity (Fleg et al., 2005; Lovejoy et al., 2008). Reduced physical activity and unbalanced dietary habits can lead to obesity by increasing fat content in the body. This promotes the secretion of various cytokines and inflammatory factors that can activate endocrine organs to secrete to adipocyte, resulting in negative (increased pro-inflammatory and decreased anti-inflammatory) changes of adipokine factors (Bruunsgaard, 2005; Freeman et al., 2010; Petersen and Pedersen, 2005). Previous studies have shown that adipokine levels are abnormal in those with obesity. It is known to be the cause of metastasis to other diseases such as cardiovascular disease, diabetes, and cancer (Perks and Holly, 2011; Roberts et al., 2010). In addition, obesity is linked to increased levels of pro-inflammatory cytokines and impaired immune systems in clinical disease (Suganami and Ogawa, 2010). Changes in lifestyle can lead to decrease in body weight. Although, such changes may not result in normal weight, they might lead to a health benefit (Wadden et al., 2007). In other words, moderate to high-intensity physical activity can result in positive changes in body compositions, including weight loss and increased lean body mass in postmenopausal (POM) women (Buonani et al., 2013a; Haskell et al., 2007).
Adipose tissue can regulate various metabolic functions as an endocrine organ. Leptin and adiponectin adipokines are known to play a pivotal role in inducing pathophysiologically important insulin resistance in metabolic and vascular-related diseases (Kwon and Pessin, 2013). High levels of leptin but low levels of adiponectin have been reported in pre- and POM women with hot flushes (Thurston et al., 2013). However, low levels of leptin but high levels of adiponectin are only found in POM women with hot flushes (Huang et al., 2017). It has been shown that exercise under hypoxic conditions is effective in increasing plasma adiponectin level and body compositions in POM women (Goldstein et al., 2004). On the other hand, the lack of physical activity can increase plasma concentrations of inflammatory factors such as interleukin (IL)-6 and tumor necrosis factor (TNF)-α but decrease insulin sensitivity and adiponectin levels (Bruunsgaard, 2005; Petersen and Pedersen, 2005). Adiponectin, an adipocyte-specific plasma protein, is known to be antiatherosclerosis and anti-inflammatory effect. It can also improve insulin sensitivity (Goldstein et al., 2004; Whitehead et al., 2006). Physical inactivity has been found to be an independent risk factor for increasing chronic low-grade systemic inflammation and its outcomes (Booth et al., 2012; Bunprajun et al., 2013; Green et al., 2014; Knudsen et al., 2012). Most POM women do not undertake regular physical exercise. However, resistance training would maximize musculoskeletal and cardiovascular therapeutic benefits for menopausal women (Williams et al., 2007).
In addition, resistance training may induce positive effects on several menopause-related diseases (Brochu et al., 2009; Zaki, 2014). Also, moderate-to-vigorous physical activity per week have lower levels of body fat and fasting glucose compared with women who undertake <150 min of moderate-to-vigorous physical activity per week (Buonani et al., 2013b; World Health Organization, 1996). There is insufficient study on changes in body composition and adipokine factors, which are regular resistance to hormonal changes during menopause. Regular resistance exercise is essential for POM women, but research is very limited. In particular, most menopausal women consider menopause a natural phenomenon. They show passive attitude toward prevention and treatment for menopause. In addition, there is a difference in hormone secretion between pre and POM women. Such may be different depending on exercise intervention. Thus, the objective of this study was to investigate effects of resistance exercise for 12 weeks on adipokine factors and body composition in obese pre and POM women to provide basic data for prevention of obesity or metabolic syndrome caused by menopausal transition.
MATERIALS AND METHODS
Subjects
Subjects of this study were 35 premenopausal (PRM) and POM women with body fat percentages of 30% or more. They were divided into PRM group (n=15, 47.5±66.11 years) and POM group (n=20, 57.79±5.68 years). The sample size of the subjects was calculated by using analysis of variance (ANOVA) a large size of effect size of 0.90, a significance level of 0.05 and a power of 0.80 (G*power 3.2.1). The results of sample size per group were calculated as 15 persons. All subjects participant in a resistance exercise program that consumed energy of 230–260 kcal daily for 12 weeks (Table 1). To be included in the present study, participants had to meet the following inclusion criteria: (a) POM (absence of a menstrual cycle for at least 1 year and follicle-stimulating hormone >30 IU/L) (Gurudut and Rajan, 2017) older than 40 (PRM) and 50 (POM) years on the date of the assessment; (b) not receiving hormone replacement treatment; and (c) not using drugs such as beta-blockers, statins, and so on. All volunteers underwent medical screening, including a health status interview and physical examination. Written informed consent was obtained from each subject. This study was approved by the Institutional Review Board of Kangwon National University (2016-04-009-002). It was conducted in accordance with the Declaration of Helsinki.
Body composition and physical fitness
All subjects underwent anthropometric measurements (height, body weight, % body fat, muscle mass, body mass index [BMI], waist-to-hip ratio [WHR]) using a multifrequency bioelectrical impedance analyzer with eight tactile electrodes (MF-BIA8) (Inbody 720 body compostion analyzer, Biospace, Seoul, Korea) at the Exercise Physiology Laboratory of Kangwon National University. Bioelectrical impedance analysis was performed after at least 8 hr of fasting and voiding. This analyzer uses an alternating current of 250 mA at a multifrequency of 1, 5, 50, 250, 500, and 1,000 kHz. It measures segmental impedances at the right arm, left arm, right, leg, left leg and trunk for all frequencies. Total body impedance value was calculated by summing segmental impedance values. Physical fitness tests were performed with a circulation measuring device using O2run’s Hellmass system 3 (grip strength, sit-ups, sit and reach test, standing long jump, and side step). All measurements were entered into an electronic card and transmitted to the computer. To measure grip strength, subjects stood with both feet at shoulder width and maintained an angle of 15 degrees so that the torso and the arm did not touch each other. They held the handle of the dynamometer with second joints of their fingers and pulled the handle while keeping their arms from shaking. For sit-ups, subjects laid on a mat and bent their knees about 140 degrees. Their feet were flat on the floor. Then the upper body was raised until elbows touched knees. The number of repetitions made in 60 sec was recorded. For sit and reach test, subjects were asked to bend their upper body while fixing the two legs in plate. For standing long jump, all subjects started with their feet in place and jumped as far as possible with the two feet landing together. For side step, parallel lines were drawn at a distance of 120 cm on the floor and subjects stood on both feet, one foot on the left side and the other foot on the right side, from the center line.
Adipokine factors test
All participants arrived at our laboratory at 7:00 a.m. After 10 min of resting in a comfortable chair, fasting blood was collected to a plain tube for serum separation from the median cubital vein after overnight (12 hr) fasting. Collected blood samples were centrifuged at 3,000 g for 10 min at 4°C and stored at −80°C freezer until appropriate analysis. All serum samples were immediately frozen and subjected to measurements with enzyme-linked immunosorbent assay for IL-6 (DY 406, R & D system Inc., Minneapolis, MN, USA), IL-15 (DY247, R & D system Inc.), leptin (DY398, R&D System Inc.), and adiponectin (DY1119, R&D System Inc.) at the Laboratory of Pharmacology by standard techniques.
Exercise intervention
Resistance exercise programs were used following experiments performed by Gurudut and Rajan (2017) with slight, modifications to fit the purpose of our experiment (Table 1). The goal of the resistance exercise intervention was to burn 230–260 kcal with moderate intensity exercise 60 min per day, 3 days per week for 12 weeks. At the beginning of each session, there was a 10 min of warm-up. It was followed by 40 min of the main part of the training with specific content and 10 min of cool-down. Warm-up exercises included 5 min of stretching, and 5 min of power walking at 50% intensity of the maximal heart rate reserve. Among resistance exercises, moderate intensity exercise was defined as circuit trainings at 55%–65% intensity of one-repetition maximum (1RM), 12 times of repeat, and 3 sets. The rest period between each category was 30 sec. The rest period between sets was 1 min in total resistance exercise time of 60 min. All exercise groups were given a polar (heart rate monitor; M400, Kempele, Finland), a portable exercise intensity setting device, for 60 min. Measurement of 1RM was calculated using formula: 1RM=lifted weight (lb)/(1.0278–repetitions×0.0278). All exercises were performed by remeasuring 1RM every 2 weeks.
In addition, dietitians and exercise physiologists met regularly with a clinical health psychologist experienced in lifestyle behavior change to discuss participant progress and refine behavior modification goals according to each participant’s needs. Nutritional education, self-management training, and behavior change techniques were provided. Furthermore, telephone consultations were scheduled biweekly for monitoring and motivation.
Statistical analysis
Data were analyzed using the IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA). Data were expressed as mean± standard deviation. All the data were tested for their normal distribution using Shapiro–Wilk test. Comparison of variables between groups before and after 12 weeks was performed by two-way ANOVA. The main analysis of interest was the ANOVA of interaction term to compare changes over time between groups. Statistical significance was accepted at α value of 0.5.
RESULTS
Results of a 12-week resistance exercise program for POM obese women are summarized in Table 2. All the results of body composition factors showed not interaction effect. Body weight, muscle mass, BMI, and WHR showed significant differences between the two groups after exercise (P<0.05). On the other hand, body fat percentage did not differ between the groups, but was significantly decreased after exercise in the PRM group (P<0.05).
In Table 3, there were significant differences between the two groups in terms of grip strength (left and right), upper body bending, sit-up, and standing long jump (P<0.05) between the two groups after exercise. In addition, grip strength (left), sit-up, and side step tests were significantly increased after exercise in the PRM group (P<0.05).
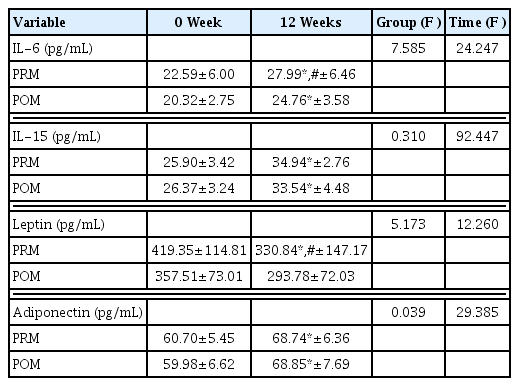
In Table 4, there was a significant difference between groups of adipokine factors, IL-6 and leptin (P<0.05). IL-6, IL-15, and adiponectin were significantly increased in both groups after exercise (P<0.05). However, leptin was significantly decreased after exercise in PRM group (P<0.05).
DISCUSSION
The lack of physical activity is considered a pivot risk factor for metabolic disease (Ekelund et al., 2015; Pedersen, 2009). The lack of physical activity is associated with a 7.5-fold higher mortality rate due to the lack of function of the immune system and obesity (Allison et al., 2012; Healy et al., 2011). On the other hand, regular exercise can decrease mortality from overweight or obesity by about 26% (Ekelund et al., 2015). Increased physical activity due to regular exercise is associated with decreased body weight and body fat (Ross and Janssen, 2001) which may reduce obesity. Results of the present study showed that regular resistance exercise in obese women before and after menopause resulted in significant changes in body fat percentage in PRM women, but not in POM women. Physical fitness such as strength, endurance, and side-step test showed significant difference in both exercise groups. These results suggest that resistance exercise is essential for pre and POM to reduce for body fat, and POM women are more required (Henríquez et al., 2017).
Adiponectin is known to be induced from visceral fat. Leptin has been reported to be secreted from subcutaneous adipose tissue (Van et al., 1998). Serum adiponectin levels are known to be reduced by abdominal obesity while serum leptin levels are highly correlated with subcutaneous fat levels (Matsuzawa et al., 1999; Weyer et al., 2001). Previous studies have shown that increased leptin and decreased adiponectin levels are directly and indirectly associated with insulin resistance, metabolic syndrome, and atherosclerosis (Beltowski, 2006). It is known that they can cause metabolic syndrome by negatively affecting glucose and lipid metabolism in menopausal transition women (Gray and Vidal-Puig, 2007). Low levels adiponectin and high leptin levels after menopausal transition can increase the risk of insulin resistance. In addition, menopause women are known have high levels of inflammatory factors such as TNF-α but low levels of adiponectin (Sites et al., 2002). Recently, the ratio of leptin to adiponectin has been considered as a powerful diagnostic tool for metabolic syndrome in menopausal transition women (Gupta et al., 2018). High physical activity has shown positive effects on decreased body fat percentage and increased adiponectin levels in women (Wang and Scherer, 2008). Regular exercise is also known to increase the level of adiponectin in women (Kobayashi et al., 2006; Lim et al., 2008). Some previous studies have reported that low-mild-intensity exercise or acute exercise is not effective in changing adiponectin levels. In other words, many studies have demonstrated no change in adiponectin level in the absence of weight or fat reduction (Simpson and Singh, 2008). Recent studies have shown that hypoxic exercise training for 8 weeks in POM women can increase the concentration of adiponectin by weight and fat reduction (Nishiwaki et al., 2016). As a result of this study, regular resistance exercise decreased leptin and adiponectin levels in both groups with decrease of body fat percentage, consistent with previous studies.
POM women with metabolic syndrome have been reported to have higher levels of IL-6 (Chedraui et al., 2014). IL-6 levels in POM women are associated with diverse metabolic syndrome factors. IL-6 level is known to be highly related to TG level (Tehrani et al., 2013). Increased levels of IL-6 may be a risk factor for cardiovascular disease in POM women. Serum IL-6 levels are associated with insulin levels and visceral fat tissue (Cartier et al., 2008). Thus IL-6 is defined as an adipokine associated with obesity or insulin resistance. It is also defined as a myokine in association with muscle contraction of skeletal muscle during exercise (Keller et al., 2001; Steensberg et al., 2000). In other words, levels of IL-6 are known to be increased after exercise without causing muscle damage. It has been reported that levels of IL-6 are related to exercise intensity, duration, endurance exercise, and muscle contraction (Febbraio and Pedersen, 2002; Pedersen et al., 2000). Another study has shown that IL-6 is expressed in muscle contraction. It is released by contraction of skeletal muscle during exercise (Hiscock et al., 2004). As a result of this study, PRM women showed positive changes in body compositions with increased muscle mass and reduced body fat percentage. In addition, IL-6 levels were increased in the same as the previous study. This suggests that resistance exercise can increased muscle contraction activity by increasing levels IL-6 which plays a role as a myokine in the contraction of muscle. The limitations of this study are that dietary control is not perfect, and its strength is motivation for regular resistance exercise to POM women.
Our results revealed that regular resistance exercise was effective in decreasing body fat percentage and increasing muscle mass in PRM women, but not POM women. It also has improved strength-related factors in physical fitness. However, adipokine factors were positively significant in both groups. That is, positive changes in adipokine factors were observed with decrease in body fat percentage and increase in muscle mass was found in PRM women. Thus, regular resistance exercise is more effective for PRM obese women than for POM obese women.
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This paper was supported by NRF -2016R1D1A1B03930331 research fund.