Effect of nutrients and exhaustive exercise on brain function
Article information
Abstract
Epidemiological evidence suggests that health-oriented eating habits are associated with maintaining optimal cognitive ability. Nutrients are functional bioactive molecules promoting human health and essential components as well. Docosahexaenoic acid (DHA; 22:6n-3), one of polyunsaturated fatty acids (PUFAs) is synthesized through elongation pathway from linolenic acid (81:3n-3) which is recognized as important source of brain function. Endurance physical exercise and energy restriction was also recognized of cardiovascular stress adjustment by enhancing brainstem cholinergic activity as well as brain function. However, we even do not know the exact neuronal mechanisms about the nutrients, β-hydroxybutyrate (β-HB) and myokine impacts on brain-derived neurotropic factor (BDNF) activation. Therefore, this review focuses on recent evidence that explains how nutrients and prolonged exercise can affect nervous system pathways that are associated with improving brain function. The results revealed that frequent consumption of polyphenols and n-3 PUFAs could modify gastrointestinal environment with beneficial microorganisms. It may suggest a new hypothesis that gastrointestinal microbiome could influence cognitive function in addition to the traditional etiological pathway. And moreover, prolonged physical exercise includes open skill sports which is induced by β-oxidation of free fatty acids stimulate BDNF. And also β-HB production which is induced by carbohydrate depletion, hypoglycemia, or fasting stimulate BDNF production that acts an significantly important roles in cognitive function and acting on brain function with brain metabolism.
INTRODUCTION
The intake of the necessary nutrients is inevitably related to the maintenance of cognition and physical fitness (Gillette-Guyonnet et al., 2013). Nutrients and bioactive molecules are essential components for human body and improve human health (Barberger-Gateau et al., 2007). Because many kinds of nutrients cannot be synthesized in our bodies, these nutrients must be ingested through various foods. For example, docosahexaenoic acid (DHA; 22:6n-3), one of polyunsaturated fatty acids (PUFAs), is synthesized through elongation pathway from linolenic acid (81:3n-3).
However, when n-3 fatty acids are lacking, DHA supply to the brain becomes insufficient. (Caracciolo et al., 2014). Fatty fish including tuna, mackerel and sardine is the major dietary sources of eicosapentaenoic acid (EPA, 20:5n-3) and DHA, as longer-chain n-3 PUAs. Enriched diet of n-3 fatty acid is known to be helpful to maintain cognitive process in human (McCann and Ames, 2005) and to upregulate expressions of many genes for maintaining synaptic function and plasticity in rodents (Wu et al., 2007). As dietary component, polyphenols are derived metabolites of plants and include flavonoids, lignans, coumarins, and tannins and appear to act on the brain in several ways (Ghosh and Scheepens, 2009; Patel et al., 2008). It has been known that frequent intake of fruits and vegetables was related with a reduced risk of all types of nervous related degeneration diseases (Barberger-Gateau et al., 2007).
Meanwhile, endurance physical exercise and energy restriction was also recognized of cardiovascular stress adjustment by enhancing brainstem cholinergic activity as well as brain function. Brain-derived neurotropic factor (BDNF) stimulation was evaluated by the persisting for a long time and repeated hypoglycemia modify multiple metabolic pathways, and also hypoglycemia which is induced by fasting or endurance physical exercise increases uptake of alternate respiratory substrates such as ketone bodies, especially the β-hydroxybutyrate (β-HB).
BDNF stimulation was also revealed by neurotrophic myokine such as insulin-like growth factor 1 (IGF-1) levels after the regular physical exercise or several kinds of exercise training. However, we even do not know the exact neuronal mechanisms about the nutrients, β-HB and myokine impacts on BDNF activation. Therefore, this review focuses on recent evidence that explains how nutrients and prolonged exercise can affect nervous system pathways that are associated with improving brain function.
NUTRIENTS FOR BRAIN FUNCTION
Accumulating studies have suggested that dietary ingredients influence mechanisms that maintain brain function (Gómez-Pinilla, 2008). Several dietary components have been proposed as health improving effects on cognitive abilities (Solfrizzi et al., 2008). Since highly chemical reactions occur in the brain, oxidative stress commonly occurs in its nervous system (Caracciolo et al., 2014). The brain is highly vulnerable to oxidative stress damage because of its metabolic load and its abundance of oxidizable materials, such as the PUFAs being consisted of the plasma membranes of neural cells. Several ‘antioxidant diets’ have become popular for their publicized positive effects on neural function (Gómez-Pinilla, 2008). Phenolic compounds, for example, have been shown to have a strong antioxidant capacity (Kelloff et al., 2000). Fruits, vegetables, plant-derived foods and beverages contain high levels of polyphenols, which are particularly abundant in colorful fruits, tea, spices, herbs, and olive oil (Chan et al., 2006; Haque et al., 2006; Kaur et al., 2008). Among polyphenols, flavonoids are the most-studied compounds related with improving brain function (Cherniack, 2012). Dietary polyphenols can play an important role in inhibiting of oxidative stress (Stevenson and Hurst, 2007). Epidemiological studies suggested that flavonoids contribute to a protective role against learning/memory defect and Alzheimer disease (Commenges et al., 2000; Letenneur et al., 2007; Schaffer et al., 2012). Supplementation with flavonoids are positively related with linguistic and verbal memory, especially with discontinuous memory as measured by the RI-48 test (Kesse-Guyot et al., 2012). Krikorian et al. (2010) provided older adults with or without mild cognitive impairment with both grape or blueberry juices for 12 weeks and found a significant improvement in spatial memory function and objective recognition memory.
N-3 PUFAs are normal components of cell membranes of brain, neuronal tissue and retina and are essential for normal brain function (Neuringer et al., 1988). PUFAs could be associated with maintaining optimal brain function and preventing against dementia by their antithrombotic and anti-inflammatory actions in addition to their specific effect on nervous functions (Gillette-Guyonnet et al., 2013). As n-3 PUFAs, DHA is a key component of neuronal membranes, and sufficient n-3 PUFA status may support maintaining optimal neuronal integrity and function. There have been known some of the mechanisms by which DHA influences neuronal plasticity and cognition. For example, dietary DHA supplementation has been found to increase levels of hippocampal BDNF and promote cognitive function in rodent models of brain trauma (Gómez-Pinilla, 2008). DHA might enhance cognitive capacities by expediting synaptic plasticity and modifying synaptic membrane fluidity. DHA may be directly associated with enhancing brain health in the aging central nervous system through a number of potential mechanisms. Moreover, DHA may moderate the expression of genes that regulate a variety of biological functions potentially important for learning/memory health (Sydenham et al., 2012).
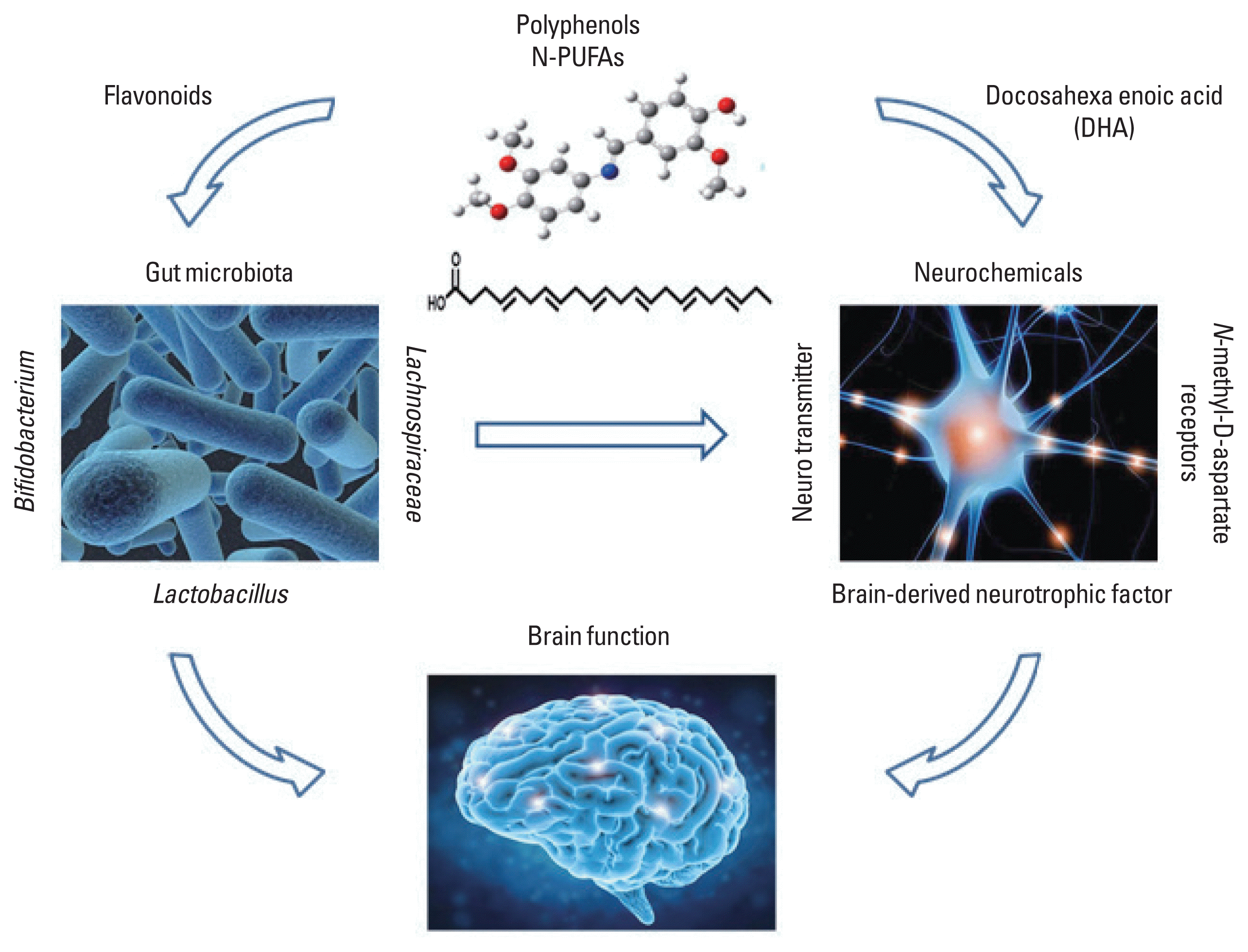
Ounnas et al. (2017) showed that intake of higher amounts of dietary polyphenols are associated with increasing EPA and DHA levels through stimulation of their synthesis in the liver, which is a major place of EPA and DHA production, suggesting that polyphenols may increase EPA and DHA levels. In addition, it has been suggested that since polyphenols and DHA have prebiotic-like effect, they can modify gut microbiota composition. Intakes of polyphenols can increase beneficial strains as Bifidobacerium and Lactobacillus, while reducing pathogenic strains such as Clostridium perfringens and C. histolyticum (Dueñas et al., 2015). Supplementation with n-3 PUFAs including DHA resulted in an increase in Bifidobacterium and butyrate-producing bacteria Lachnospiraceae family and a decrease in Faecalibacterium (Costantini et al., 2017). The connection between microbiota in gastrointestinal system and the central nervous system plays an important role in maintaining optimal brain function (Filosa et al., 2018). The gut microbiota can synthesize neurotransmitters including γ-aminobutyric acid and serotonin, and regulate their levels acting on their precursors (O’Mahony et al., 2015). The recently proposed microbiome-gut-brain axis (Biagi et al., 2010; Claesson et al., 2012) is gaining recognition for its potential role not only in gastrointestinal disorders, but also in diseases of the central nervous system. Thus, frequent consumption of polyphenols and n-3 PUFAs could modify gastrointestinal environment with beneficial microorganisms. It may suggest a new hypothesis that gastrointestinal microbiome could influence cognitive function and the traditional etiological pathway as well.
EFFECT OF EXERCISE ON BRAIN FUNCTION
It was well recognized that upregulated BDNF is associated with the neuronal plasticity which was induced by different kinds of physical exercise and intermittent fasting.
Ketone bodies, especially the β-HB as the upstream effector of BDNF were enhanced by prolonged exercise, different kinds of fasting, hypoglycemia and diabetes mellitus. Previous research revealed ketone body production which was triggered by prolonged physical exercise such as marathon, triathlon racing and energy restriction (Lan et al., 2018).
Prolonged physical exercise and energy constraint also augment cardiovascular stress adjustment by enhancing brainstem cholinergic activity as well as brain function (Rothman et al., 2012). Ketone bodies can be the most efficient energy source in almost every tissue and have more adenosine triphosphate production per mole of energy substrate than pyruvate. Persisting for a long time and recurrent hypoglycemia regulate multiple metabolic pathways, and also hypoglycemia which is induced by fasting or prolonged physical exercise increases uptake of alternate respiratory substrates such as ketone bodies (Rehni and Dave, 2018).
The metabolism of ketone bodies interfaces with the tricarboxylic acid cycle, β-oxidation of free fatty acids, de novo lipogenesis, sterol biosynthesis, and ATP productions at glucose presents from important metabolic intermediates, acetyl CoA (Cotter et al., 2013).
β-HB protect neurons against many oxidative stresses and enhances mitochondrial respiration heading to enhanced expression of BDNF (Park and Kwak, 2017). From the physical exercise point of view, crucial recent research paper indicate that strength exercise inhibits aerobic exercise-induced cognitive improvements and adult hippocampal neurogenesis (Lan et al., 2018).
This paper also emphasis on the β-HB production which is induced by β-oxidation of free fatty acids versus carbohydrate oxidation during prolonged exercise. This kind of exercise connected with the enhanced spatial learning and memory function as well as hippocampal neurogenesis. Hippocampal neurogenesis is also well connected mechanisms with the exercise-induced neuronal synaptic plasticity. However, strength exercise is mainly anaerobic exercise that uses glycolysis, this anaerobic exercise decreased these benefits (Lan et al., 2018).
Open skill exercise, such as badminton, showed meaningful higher serum BDNF levels and excellent executive function compare to the closed skill exercise, such as running (Hung et al., 2018).
Another pathway of BDNF stimulation was also revealed by neurotrophic myokine, such as IGF-1, following regular physical exercise or several kinds of exercise training (Kwak and Lim, 2018). BDNF acts gratefully important roles in cognitive function and acting on brain function include brain metabolism. And this also important roles in the integration and optimization of metabolic responses to some situations and severe exercise events (Rothman et al., 2012).
Emerging evidence clarified that BDNF is a primary regulator of synaptic transmission and synaptic plasticity in adult synapses in many areas of central nervous system. And it is linked to the regulation of adult hippocampal neurogenesis. And BDNF-TrkB signaling is closely connected to neuronal development (Donovan et al., 2008).
CONCLUSIONS
Frequent consumption of polyphenols and n-3 PUFAs can modify gastrointestinal environment by increasing beneficial microorganisms. Gastrointestinal microbiome might influence cognitive function in addition to traditionally suggested etiological pathways. Based on the present our studies, it can be suggested that prolonged physical exercise, such as open skill sports, causes β-oxidation of free fatty acids, and then stimulates BDNF production. β-HB production induced by carbohydrate depletion, hypoglycemia or fasting also stimulates BDNF production. Enhanced BDNF production improves cognitive function through facilitation of brain metabolism. BDNF also acts as primary regulator of synaptic transmission, synaptic plasticity, and hippocampal neurogenesis in the many areas of central nervous system.
It may suggest a new hypothesis that gastrointestinal microbiome could influence cognitive function and the traditional etiological pathway as well (Fig. 1).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
ACKNOWLEDGMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT and Future Planning (NRF-2017R1A2B4005915).