Impact of long-term high-intensity interval and moderate-intensity continuous training on subclinical inflammation in overweight/obese adults
Article information
Abstract
Obesity is a risk factor able to trigger several inflammatory alterations and the imbalance between pro- and anti-inflammatory cytokine productions. Physical exercise is an important strategy for reduction of inflammatory established process. The aim of this study was to evaluate the effect of 16 weeks of three exercise training programs in the inflammatory profile and insulin resistance in overweight/obesity. Thirty two men and women (46.4±10.1 years; 162.0±9.1 cm; 82.0±13.6 kg) were divided into three groups for training on a treadmill: continuous at 70% maximum heart rate (HRmax) 5 times a week (CONT); 1×4 min (1-bout) and 4×4 min (high intensity interval training, HIIT) at 90% HRmax 3 times a week. Interleukin (IL) 6 and IL-10, tumor necrosis factor-alpha (TNF-α), insulin and adiponectin levels were analyzed by enzyme-linked immunosorbent assay, and homeostasis model assessment insulin resistance was calculated. After 16 weeks of training blood concentrations of IL-6 decreased in the HIIT group (P=0.035), TNF-α decreased in the CONT (P=0.037) and increased in HIIT (P=0.001) and adiponectin decreased in the three training models. There was a trend towards decreased body weight and body mass index (BMI) after HIIT only (P=0.059 and P=0.060, respectively). Despite the decrease of adiponectin and the increase of TNF-α in HIIT group, insulin sensitivity showed a trend for improvement (P=0.08). HIIT program decreased IL-6 at rest and although not significant was the only who tended to decrease total body weight and BMI. Taken together, our data suggest that both HIIT as well as CONT exercises training program promotes changes in inflammatory profile in overweight/obesity, but dissimilar response is seen in TNF-α levels.
INTRODUCTION
Obesity is a risk factor able to trigger several inflammatory alterations favorable for the chronic low-grade inflammation, by imbalance between pro- and anti-inflammatory cytokine productions. Furthermore, obesity is positively associated with installation and development of diseases as type 2 diabetes, cardiovascular diseases and metabolic syndrome in populations of different ages and genders (Weghuber et al., 2014; Wen et al., 2013).
Chronic low-grade inflammation observed in illness associates with modifications in plasmatic cytokines concentration, as high concentrations of plasminogen activator inhibitor-1 (Lira et al., 2010), C-reactive protein, interleukin (IL) 6 and tumor necrosis factor-alpha (TNF-α), which are considered pro-inflammatory biomarkers. In addition, this inflammatory context reduces anti-inflammatory cytokine concentration, as adiponectin, and IL-10. Obese individuals have low concentrations of adiponectin, and this is associated with the metabolic syndrome (Renaldi et al., 2009), type 2 diabetes (Hotta et al., 2000), dyslipidemia (Lara-Castro et al., 2006) and coronary artery diseases (Vilarrasa et al., 2005).
Several studies have documented the effectiveness of exercise training on metabolic disorders, prevention and treatment, and its anti-inflammatory effect (de Meirelles et al., 2014; Karstoft and Pedersen, 2016; Lira et al., 2015). High intensity interval training (HIIT) has been proposed to be a time-efficient (low volume) method to improve aspects related to body composition and disease (Madsen et al., 2015a; Madsen et al., 2015b; Sijie et al., 2012; Talanian et al., 2007). Madsen et al. (2015a) showed only a modest improvement in anti-inflammatory cytokine after eight weeks of HIIT for subjects at risk for metabolic syndrome individuals, while inflammatory cytokines did not change. On the other hand, have observed that HIIT is more effective to treat excessive body weight than moderate continuous training (Sijie et al., 2012). These differences in results may be due to differences in training intensity and volume, and training length (8 weeks) (Madsen et al., 2015a). Thus, whether longer than 8-week HIIT, with lower volume than typical training session can improve metabolic and inflammatory profile of subjects with overweight and obesity is unknown. Therefore, the purpose of the present study was (a) analyze the effects of 16 weeks of different models exercise training (high-intensity interval vs. moderate-intensity continuous training) on inflammatory profile; and (b) analyze the effects of 16 weeks of two HIIT protocols (1-bout 1×4 min vs. HIIT 4×4 min) on metabolic and inflammatory profile of subjects with overweight and obesity.
MATERIALS AND METHODS
Participants
Subjects with overweight and obesity (body mass index≥25 kg/m2) were invited to participate in the study through dissemination of the project in social networks, printed posters, emails list from students and employees at the University of Sao Paulo - Campus of Ribeirao Preto, patients list of Hospital’s Ribeirao Preto Medical School, University of São Paulo, radio, and television stations.
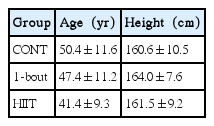
In this study, subjects of both genders (women and men), aged 18 years old or more, was randomized and stratified into three groups: HIIT 3 times a week, 4×4 min (HIIT); “one bout” training 3 times a week, 1×4 min (1-bout); and continuous training (CONT) (30 min, 5 times a week). The criteria for exclusion were: unstable angina, recent heart attack (4 weeks), decompensated heart failure, severe valvular disease, uncontrolled hypertension, renal failure, orthopedic/neurological limitations, cardiomyopathy, presence of surgeries planned during the study period, reluctance to sign the consent form and informed participation in another study, and alcohol or drug abuse. The characteristics of each group at baseline are presented in Table 1.
Training program
HIIT and 1-bout programs were carried out by walking/running 3 times a week on a treadmill. Warming up consisted of 10 min at 70% of maximum heart rate (HRmax) (13 on Borg scale of 6 to 20 points). Afterwards subjects performed four (HIIT) or a unique (1-bout) period of four minutes at 90% of HRmax (16 on Borg scale). On the HIIT session the bouts were interspersed by 3 min of active recovery (70% of HRmax). Finally, both groups, there were 5 min cool down. The CONT corresponded to 70% of HRmax (13 on Borg scale) for 30 min, 5 times a week. The duration of all three training programs was 16 weeks being the faults recorded and heart rate monitored continuously during the sessions supervised to ensure that the subjects exercised at the correct intensity (Tjønna et al., 2008).
Immunoassays for cytokines
Blood samples was collected by venipuncture in the antecubital fossa and collected 10 mL of blood that were centrifuged at 3,000 rpm for 8 min at 4°C to separated serum and plasma, and then stored in aliquots at −80°C for future analysis. Analysis of cytokines were performed by Enzyme immunoassay ELISA (enzyme-linked immunosorbent assay) using a microplate reader by SpectraMax Plus 384 Absorbance Microplate Reader (San Diego, CA, USA) with a 450 nm filter for reading absorbance.
Analysis of TNF-α, IL-6 and adiponectin concentrations was performed using reagents kits from R&D System (R&D System Inc, Minneapolis, MN, USA) with sensitivity of 1,000 to 15.6 pg/mL, 300 to 4.7 pg/mL, and 4,000 to 62.5 pg/mL, respectively. The intra-assay variability of the TNF-α kit was 4.2 to 5.2%; IL-6 kit was 1.6 to 4.2% and adiponectin kit was 0.6 to 6.0%.
For the analysis of circulating IL-10 concentrations the reagent kits from eBioscience (Affymetrix Inc,, San Diego, CA, USA) was used, with sensitivity of 300 to 2.3 pg/mL, intra-assay variability was 0.3 to 1.0%. Analysis of insulin concentrations was performed using reagents kits from Accubind (Monobind Inc., Lake Forest, CA, USA) with sensitivity of 0 to 300 μlU/mL, intra-assay variability was 4.3 to 8.3%.
Statistical analysis
Data normality was verified by the Shapiro–Wilks test. To evaluate the effect of training the Student t-test was applied between pre and post 16 weeks in each group. To verify possible differences between baseline (pretraining), as well as the magnitude of the variations (Δ) after training among the three groups we used the one-way analysis of variance. For all analyzes, we used the SPSS ver. 13.0 (SPSS Inc., Chicago, IL, USA). A significance level of 5% was adopted.
RESULTS
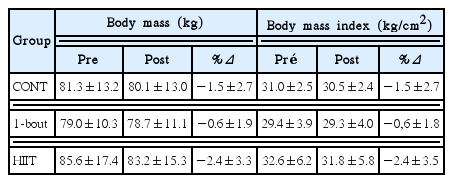
The modification of total body mass and body mass index before and after training are presented in Table 2. None group promoted significant changes in body composition of participants, however the HIIT group tended to reduce the total body weight and BMI (P=0.059 and P= 0.060, respectively).
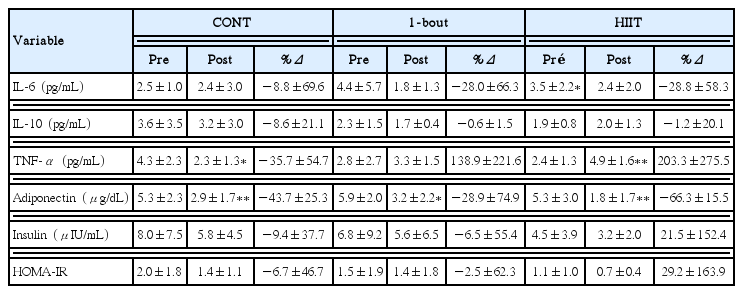
Blood measurements of plasma cytokines and insulin are shown in Table 3. In HIIT group IL-6 concentrations decreased (P= 0.035) in contrast to TNF-α that increased (P=0.001) with training. In CONT group TNF-α blood values reduced (P=0.037) after the training period. The plasma concentrations of adiponectin decreased significantly in all three groups (P=0.009, P=0.022, and P=0.0002 in CONT, 1-bout, and HIIT, respectively), but there was no difference in magnitude (Δ%) of these changes between group (Δ% CONT=−43.7±25.6; Δ% 1×4 min=−28.9± 74.9; Δ%=−66.3±15.5 HIIT). With the exception of adiponectin, the 1-bout training did not cause any other changes in the evaluated parameters. IL-10, insulin, and homeostasis model assessment insulin resistance (HOMA-IR) did not change across the three training models.
DISCUSSION
The aim of the present study was to evaluate the effect of 16 weeks of different models of exercise training on metabolic and inflammatory profile of subjects with overweight and obesity. The main findings were that 16 weeks of training on a treadmill (a) reduced IL-6 in the HIIT-4×4 min group, (b) increased TNF-α in HIIT-4×4 min group and reduced it in CONT, and (c) reduced adiponectin levels in all groups.
Exercise causes great changes in cellular energy status and immune system. The elevated IL-6 production by skeletal muscle during training sessions performs important functions for auxiliary energy supply, stimulating lipolysis in situations of high adenosine monophosphate/adenosine triphosphate ratio and low glycogen stores (Pedersen, 2012). It also acts on anti-inflammatory form, promoting IL-10 production, which in turn inhibits the action of nuclear factor kappa B, and therefore the synthesis of pro-inflammatory cytokines such as TNF-α and interleukin-1β (Galic et al., 2010).
In a recent study Cabral-Santos et al. (2015) showed that IL-6 concentrations are significantly elevated after high intensity intermittent stimulus (5 km performed 1:1 100% of VO2peak [peak oxygen consumption]) when compared to continuous moderate intensity. This may be due to increased demand for glucose, as evidenced by the high blood lactate concentrations usually found in these training models. Tschakert et al. (2015) reported that in a protocol in cycle ergometer (4×4 min - 95% of HRmax) similar to the present study HIIT the blood lactate concentrations and respiratory exchange ratio were significantly higher than in the lower intensity continuous. These data indicate the highest metabolic stress generated in HIIT favoring greater IL-6 production.
Unlike the exercise, at rest approximately 30% of circulating IL-6 comes from the adipose tissue and that total two-thirds of infiltrated macrophages (Fried et al., 1998; Mohamed-Ali et al., 1997). It is important to consider that the IL-6 is also considered to be the main inducer of the expression of several proteins that play an important role on inflammatory status. High plasma concentrations of IL-6 together with C-reactive protein, fibrinogen, and amyloid A are related to chronic low-grade inflammation and involved with the development of some diseases, such as diabetes, atherosclerosis, and rheumatoid arthritis (Reihmane and Dela, 2014). Thus, the present study showed that 16 weeks of HIIT training reduced IL-6 concentration in overweight/obeses subjects. This result can be understood as a supportive and protective adaptation to these diseases.
Obesity is associated with a sedentary lifestyle and high caloric intake. These lifestyle habits promote the adipocyte hypertrophy, which decreases blood and oxygen supply to the adipose tissue. This chronic and progressive reduction stimulates the recruitment of monocytes and secretion of pro-inflammatory cytokines, such as IL-6 and TNF-α (Gleeson et al., 2011). TNF-α is a cytokine produced primarily by monocytes and macrophages infiltration in adipose tissue. However, other immune cells, such as lymphocytes and natural killer cells, may also produce it. High concentration of TNF-α is usually associated with cell death, cardiovascular diseases, inflammation and other acute phase proteins (Golbidi and Laher, 2014). This cytokine also activates certain intracellular kinases, which inhibit the signaling of insulin, impairing glucose uptake (Diehl, 2004).
The Leggate et al.’s (2012) study with overweight/obese men showed that 2-week HIIT not alter the blood concentrations of IL-6, IL-10, and TNF-α. On the other hand, adipose tissue concentrations of monocyte chemoattractant protein-1, IL-10, and TNF-α were undetectable after training, indicating beneficial adaptations in the resting inflammatory profile. Robinson et al. (2015) evaluated the effects of HIIT and continuous in the same population (predominantly obese women) and also found an improvement in inflammatory profile indicated by the decrease of Toll-like receptors type 4 (lymphocytes and monocytes) and type 2 (lymphocytes), membrane receptors known to be related to inflammatory response, even without changes in blood cytokines.
In light of this knowledge, the reduction of circulating TNF-α after 16 weeks of CONT training can be beneficial, but we cannot claim that the increase in circulating TNF-α post HIIT is harmful because it is known that blood concentrations can not accurately reflect the intracellular and tissue reality. In addition these changes did not alter the concentrations of insulin and HOMA-IR values, suggesting that insulin sensitivity was not affected by different concentrations of TNF-α.
Another issue to consider is that the action of TNF-α, as well as its efficiency, are dependent on their receivers. In cell membrane there are two types of receptors (TNFR1 and 2) and pro-inflammatory characteristics generally associated with the protein (mainly in adipose tissue) are past their binding to TNFR1. These receptors may also be cleaved and become soluble in plasma (sTNFR) and thus act in on anti-inflammatory form, binding to TNF-α and prevent its connection to the cell membrane and subsequent signal transduction (Cawthorn and Sethi, 2008; Gatanaga et al., 1990; MacEwan, 2002). So, if TNF-α post HIIT increases are accompanied by the increase of sTNFR, the functions of this protein can be suppressed. More studies are need for better understanding of mechanism involved.
Another variable analyzed was adiponectin. Studies showed that it is reduced in situations of obesity and insulin resistance when compared to healthy individuals and animals (Hu et al., 1996; Weyer et al., 2001). Weight loss and exercise are common treatments for improvement in insulin sensitivity, but Hulver et al. (2002) showed that only the weight loss is effective for increased in the adiponectine levels. Suggesting that exercise training improves insulin sensitivity by independent mechanisms for weight loss and adiponectin action. Madsen et al. (2008) showed that an increase in adiponectine levels requires a reduction of at least 10% in total body mass. Another study by Christiansen et al. (2010) found conflicting results showing augment of adiponectin only in the groups that performed restrictive diet and diet coupled with aerobic exercise for 12 weeks. In this study there was also a third group that performed only exercises without calorie restriction, with smaller reduction in fat mass and no significant decrease in adiponectine levels.
A review by Simpson and Singh (2008) showed that only three of eight studies with exercise increased adiponectine levels. Despite the benefits of this adipokine in increased insulin sensitivity, it is important to note that there are different isoforms of adiponectin with not entirely clear functions, and exercise seems to regulate differently each isoform. Other studies may want to verify whether different exercise intensity modulates adiponectin.
ACKNOWLEDGMENTS
Fabio Santos Lira thanks Fundaçao de Amparo à Pesquisa do Estado de Sao Paulo - Fapesp for their support (2013/25310-2).
Notes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.