INTRODUCTION
Increasing effect of physical exercise on neurogenesis in the hippocampal dentate gyrus has been well documented (Baek et al., 2012; Cho et al., 2013; Kim et al., 2003; Kim et al., 2010; Kim et al., 2014). Hippocampal adult neurogenesis is closely associated with hippocampal functions, such as learning ability and memory function (Baek et al., 2012; Kim et al., 2010; Kim et al., 2014). Neurogenesis in the hippocampus is enhanced by physical exercise, and this enhancement of neurogenesis is considered as the underlying mechanism of exercise-induced improvement of many neuropsychiatric disorders (Cho et al., 2013; Kim et al., 2013; Kim et al., 2014; Seo et al., 2013).
Brain-derived neurotrophic factor (BDNF) is widely distributed in the adult brain and BDNF is involved in the activity-dependent neuronal plasticity and synaptic transmission (Calabrese et al., 2009; Poo, 2001). BDNF is a small dimeric protein, and works through high affinity binding with its receptor, tyrosine kinase B (TrkB). BDNF enhances hippocampal-dependent memory and long-term potentiation via TrkB (Lu et al., 2008). Klintsova et al. (2004) reported that physical activity increased BDNF and TrkB expression in the motor cortex. Reduced BDNF level is implicated in the progression of neurodegenerative diseases, such as Huntington disease, Alzheimer disease, and Parkinson disease (Kim et al., 2014; Nagahara and Tuszynski, 2011; Zuccato and Cattaneo, 2007).
The effect of exercise, which increases hippocampal neurogenesis and improves memory function, is well documented, however, differences in the effect of exercise on young children and adults are not yet known. In the present study, we comparatively investigated the differences of treadmill exercise on spatial learning ability between young- and adult-age rats. For this study, immunohistochemistry for 5-bromo-2′-deoxyuridine (BrdU) and Western blot for BDNF and TrkB were performed.
MATERIALS AND METHODS
Animals
Adult male Sprague-Dawley rats, weighing 300±10 g (20 weeks old) for adult-age groups, and young male Sprague-Dawley rats, weighing 120±5 g (5 weeks old) for young-age groups were obtained from commercial breeder (Orient Co., Seoul, Korea). The experimental procedures were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. The rats were housed under controlled temperature (20°C±2°C) and lighting (7:00 a.m. to 7:00 p.m.) conditions with food and water available ad libitum. The rats were divided into 4 groups (n=10 in each group): young-age control, young-age exercise, adult-age control, adult-age exercise groups. All rats received 50-mg/kg BrdU (Sigma Chemical Co., St. Louis, MO, USA) intraperitoneally, once a day 30 min before the starting of treadmill exercises for 6 consecutive weeks.
Exercise protocol
The rats in the exercise groups were forced to run on a motorized treadmill for 30 min once a day for 6 weeks. The exercise load consisted of running at a speed of 2 m/min for the first 5 min, 5 m/min for the next 5 min, and 8 m/min for the last 20 min, with a 0° inclination. The rats in the control groups were left on the treadmill without running for the same period as the exercise groups.
Radial 8-arm maze test
Spatial learning ability was tested using a radial 8-arm maze apparatus, as the previously described method (Kim et al., 2010; Seo et al., 2013). The radial 8-arm maze apparatus consisted of a central octagonal plate (30 cm in diameter) and radiating eight arms (50 cm in length and 10 cm in width). The apparatus was placed 1 m above the floor. A small receptacle filled with water was located at the end of the arms. The rats were trained 3 times before the spatial learning test. The rats were deprived of water for 24 hr and were allowed to explore the water for 5 min. The test was conducted immediately after the last treadmill exercise. The time spent in seeking water at the end of the arms was counted. The test was finished when rats found water in all eight arms or over 5 min elapsed. Reentry into the previously visited arms was counted as an error.
Tissue preparation for immunohistochemistry
The rats were sacrificed immediately after determining the latency time of the radial 8-arm maze test, as the previously described method (Kim et al., 2010; Seo et al., 2013). The animals were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneally; Vibac Laboratories, Carros, France), transcardially perfused with 50-mM phosphate-buffered saline (PBS), and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100-mM phosphate buffer (pH, 7.4). The brains were dissected and postfixed in the same fixative method overnight, and transferred to a 30% sucrose solution for cryoprotection. Forty-micrometer-thick coronal sections were made using a freezing microtome (Leica, Nussloch, Germany). On average 10 slice sections in the hippocampus were collected from each rat. The sections obtained 2.5–2.7 mm posterior to the bregma were used for immunohistochemistry.
BrdU immunohistochemistry
To detect newly generated cells in the dentate gyrus, BrdU-specific immunohistochemistry was performed, according to the previously described method (Kim et al., 2013; Seo et al., 2013). The sections were first permeabilized by incubation in 0.5% Triton X-100 in PBS for 20 min, then pretreated in 50% formamide-2 × standard saline citrate at 65°C for 2 hr, denatured in 2 N HCl at 37°C for 30 min, and rinsed twice in 100-mM sodium borate (pH, 8.5). Afterwards, the sections were incubated overnight at 4°C with BrdU-specific mouse monoclonal antibody (1:600; Roche, Mannheim, Germany). The sections were then washed three times with PBS and incubated with biotinylated mouse secondary antibody (1:200; Vector Laboratories, Burlingame, CA, USA) for 1 hr. The sections were then incubated for another 1 hr with an avidin-peroxidase complex (1:100; Vector Laboratories). For visualization, sections were incubated in 50-mM Tris-HCl (pH, 7.6) containing 0.03% 3,3′-diaminobenzidine (DAB), 40-mg/mL nickel chloride, and 0.03% hydrogen peroxide for 5 min.
After BrdU staining, the differentiation of BrdU-positive cells was determined on the same section using a mouse anti-neuronal nucleic antibody (1:1,000; Chemicon International, Temecula, CA, USA). The sections were washed 3 times with PBS, incubated for 1 hr with a biotinylated anti-mouse secondary antibody. For staining, the sections were incubated in a reaction mixture consisting of 0.03% DAB and 0.03% hydrogen peroxide for 5 min. The sections were mounted onto gelatin-coated slides, air0dried overnight at room temperature, and coverslips were mounted using Permount (Fisher Scientific, New Jersey, NJ, USA).
Western blot analysis
BDNF and TrkB expressions were determined by Western blotting, according to the previously described method (Kim et al., 2013; Kim et al., 2014). The hippocampal tissues were collected, and then were immediately frozen at −70°C. The hippocampal tissues were homogenized on ice, and lysed in a lysis buffer containing 50-mM HEPES (pH, 7.5), 150-mM NaCl, 10% glycerol, 1% Triton X-100, 1-mM phenylmethylsulfonyl fluoride, 1-mM ethylene glycol tetraacetic acid, 1.5-mM MgCl2·6H2O, 1-mM sodium orthovanadate, and 100-mM sodium fluoride. Protein content was measured using a Bio-Rad colorimetric protein assay kit (Hercules, CA, USA). Protein (30 μg) was separated on sodium dodecyl sulfate-polyacrylamide gels and transferred onto a nitrocellulose membrane. Mouse actin antibody (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit BDNF antibody (1:1,000; Santa Cruz Biotechnology), and rabbit TrkB (1:1,000; Santa Cruz Biotechnology) were used as the primary antibodies. Horseradish peroxidase-conjugated anti-rabbit antibody for BDNF (1:2,000; Vector Laboratories) and TrkB (1:3,000; Vector Laboratories) were used as the secondary antibodies.
Experiments were performed in normal laboratory conditions and at room temperature, except for the transferred membranes. Transferred membranes were performed at 4°C with the cold pack and prechilled buffer. Band detection was performed using the enhanced chemiluminescence detection kit (Santa Cruz Biotechnology).
Data analysis
For confirming the expressions of BDNF and TrkB, the detected bands were calculated densitometrically using Molecular Analyst, version 1.4.1 (Bio-Rad). The numbers of BrdU-positive cells in the dentate gyrus were counted hemilaterally under a light microscope (Olympus, Tokyo, Japan). The area of the dentate gyrus was measured by Image-Pro Plus image analysis system (Media Cyberbetics Inc., Silver Spring, MD, USA). The numbers of BrdU-positive cells were expressed as the numbers of cells per mm2 in the hippocampal dentate gyrus.
The data were analyzed with one-way analysis of variance and then Duncan post hoc tests. All values are expressed as the mean± standard error of the mean. P<0.05 was considered as significant.
RESULTS
Effect of treadmill exercise on spatial learning ability
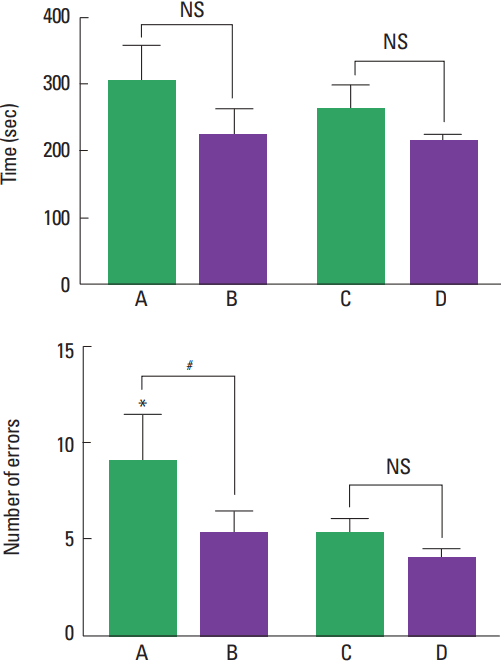
The results of the radial arm maze test are presented in Fig. 1. The present results show that the number of error was higher in the young-age rats compared to the adult-age rats (P<0.05). Treadmill exercise decreased the number of error in the young-age rats (P<0.05).
Effect of treadmill exercise on neurogenesis
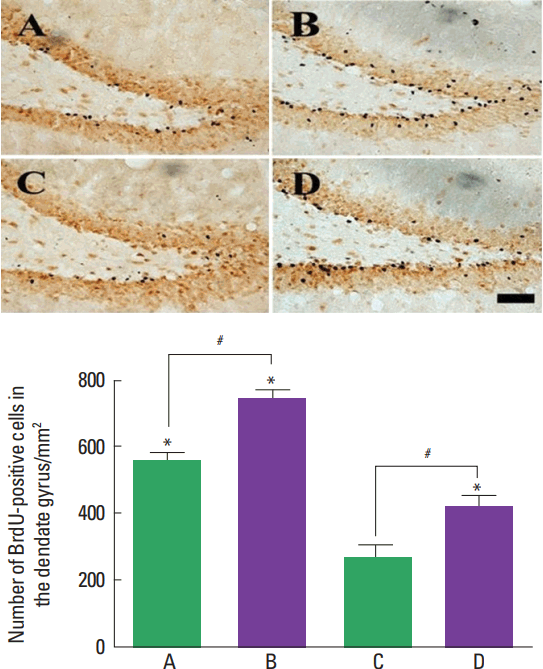
Photomicrographs of BrdU-positive cells in the hippocampal dentate gyrus are presented in Fig. 2. The present results show that neurogenesis occurred more actively in the young-age rats compared to the adult-age rats (P<0.05). Treadmill exercise increased neurogenesis in both young- and adult-age rats (P<0.05).
Effect of treadmill exercise on BDNF expression
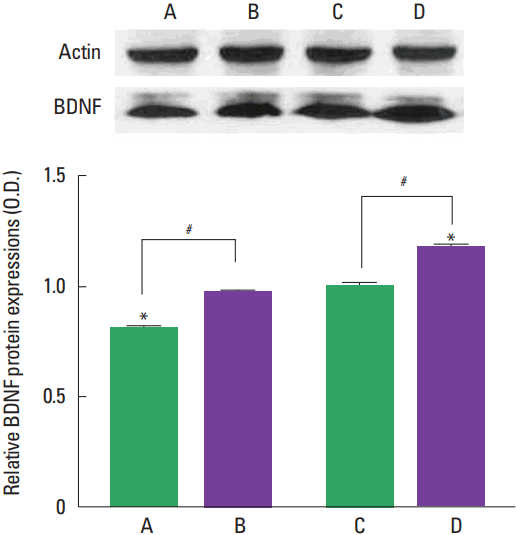
Photomicrographs of BDNF expression in the hippocampal dentate gyrus are presented in Fig. 3. The present results show that BDNF expression was higher in the adult-age rats compared to the young-age rats (P<0.05). Treadmill exercise increased BDNF expression in both young-age rats and adult-age rats (P<0.05).
Effect of treadmill exercise on TrkB expression
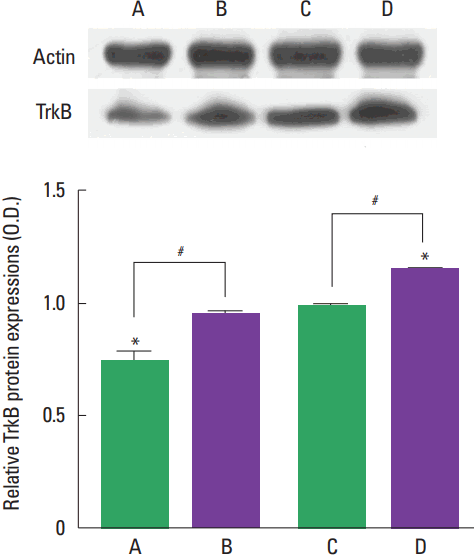
Photomicrographs of TrkB expression in the hippocampal dentate gyrus are presented in Fig. 4. The present results show that TrkB expression was higher in the adult-age rats compared to the young-age rats (P<0.05). Treadmill exercise increased TrkB expression in both young-age rats and adult-age rats (P<0.05).
DISCUSSION
In general, people think that when they are young, they have better learning ability than adults. Richards et al. (2004) investigated the relationship between childhood cognitive function and mid-life cognitive decline in a normal population. They suggested that cognitive ability in childhood was significantly and negatively associated with cognitive decline in mid-life and beyond (Richards et al., 2004). Fors et al. (2009) suggested the importance of childhood living conditions in maintaining cognitive function even in late life.
Aerobic exercise may relate with hippocampal volume and memory during childhood (Chaddock et al., 2010). Aerobic exercise selectively affects hippocampal function through increasing angiogenesis, synaptogenesis, and neurogenesis (Baym et al., 2014). In the present study, the number of error in the young-age rats was effectively decreased by treadmill exercise (Fig. 1). The present results suggest that improvement of spatial learning ability by treadmill exercise appeared in children rather than in adults.
Hippocampal neurogenesis is closely associated with improvement of learning ability and memory function under the various brain disorders (Baek et al., 2012; Chen et al., 2006; Kim et al., 2010; Kim et al., 2014; Seo et al., 2013). Treadmill exercise delayed cognitive decline by the enhancing neurogenesis and increasing BDNF expression in the vascular dementia rats (Choi et al., 2016). In the present study, hippocampal neurogenesis was more active in the young-age rats than in the adult-age rats. The effect of treadmill exercise on neurogenesis was enhanced in both young- and adult-age rats (Fig. 2).
BDNF-TrkB signaling plays a fundamental role from acquisition to consolidation in the hippocampal-dependent learning and memory (Tyler et al., 2002). Exercise-induced increase in the hippocampal BDNF level might be the underlying mechanism of exercise enhancing synaptic plasticity and cognitive function (Vaynman et al., 2003; Vaynman et al., 2004). Brain BDNF was gradually increased from the postnatal to maturation but not in old rats (Karege et al., 2002). The levels of BDNF and TrkB isoforms in the hippocampus showed age-related differences (Silhol et al., 2007). BDNF mediates activity-dependent processes, such as neuronal differentiation and growth, in the mammalian brain (Park and Poo, 2013). BDNF-TrkB signal enhances hippocampal neurogenesis (Wei et al., 2015), and upregulation of BDNF and TrkB is closely related to the exercise-induced improvement of hippocampal-dependent memory (Liu et al., 2008; Maass et al., 2016). In the present study, BDNF and TrkB expression in the hippocampus was greater in the adult-age rats than in the young-age rats. The effect of treadmill exercise on BDNF and TrkB expression was increased in both young-age rats and adult-age rats (Figs. 3, 4).
The results of this study showed that adults have excellent spatial learning abilities than children, but the improvement of exercise-induced spatial learning ability through neurogenesis is better in children. Therefore, exercise during childhood is expected to have a better effect on improving learning ability and memory function.